41 lewis dot diagram for methane
lewis dot ch4 diagram structure methane electron number o2 chemistry total electrons valence draw molecule oxygen dioxygen gas calculate How to Draw the Lewis Dot Structure for CH4: MethaneA step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use...
Lewis Dot Diagram Methane Wiring Schematic Diagram The following is a 3 d lewis structure for methane ch 4. Electron dot diagram for methane. The lewis structure does not give a real 3 dimensional structure of a molecule but it is a really good first attempt. Lewis dot dragram for methane.

Lewis dot diagram for methane
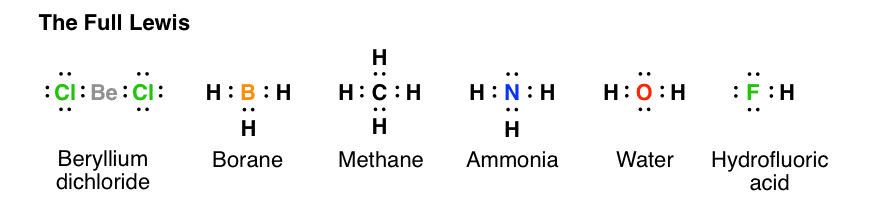
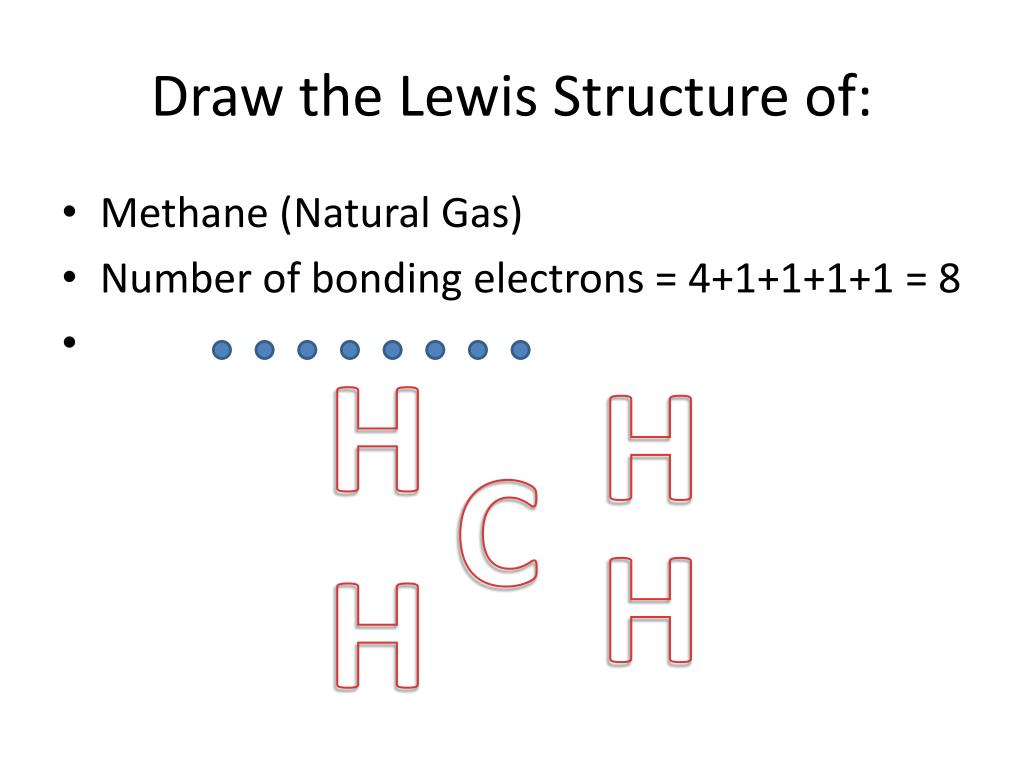
CH4 Lewis Structure. Answer: CH4 Lewis structure (Methane electron dot structure) is that type of diagram where we show the total 8 valence electrons of CH4 as dots , or dots and dashes (-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash (-) or dots ( ) but a lone pair of two electrons is shown by dots [ ]. Lewis Dot Structures can be produced by following a sequence of steps. Let's produce a Lewis Dot Structure for: NH 4 + (the ammonium ion). Step 1: Count valence electrons: N = 5 4 x H = 4 x 1 = 4 "+" = -1 Total = 5+4-1= 8 electrons = 4 bonds and lone pairs. Step 2:!Arrange the atoms (identify a central atom, if possible). Lewis Dot Structure for CH4 (Methane) Properties of methane are described by Lewis Structure as cheaper natural gas than electricity. Methane, or CH4, is a natural gas that is relatively plentiful on earth, making it an environmentally effective source.
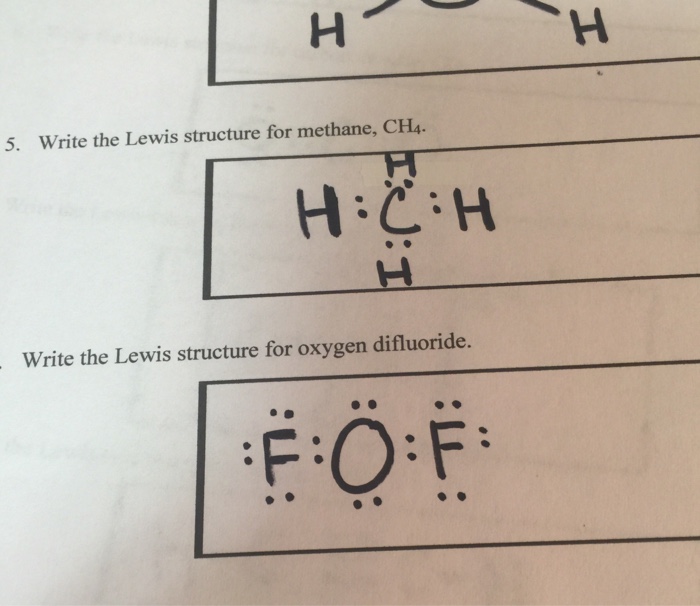
Lewis dot diagram for methane. Ch4 Electron Dot Diagram. I will explain this with pictures, and some captions. This is just the five atoms in CH4, or Methane. I have drawn them above. The red one in the. Lewis Dot Structure for CH4 #2 Find the number of "octet" electrons for the molecule. C: 8 octet electrons x 1 atom = 8 octet electrons H: 2 octet electrons x 4 . Hydrogen only has one valence electron and can only share one. Methane's (When it comes to hydrocarbons, “meth” stipulates one carbon, “ane” stipulates a single ...1 answer · 4 votes: Well Carbon only has 4 valence electron, so it can bond at all four point. Hydrogen only ... The Lewis structure of the methane (CH4) molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each. Moreover, as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals, the hybridization of CH4 is sp3. Drawing the Lewis Structure for CH 4. For CH 4 you have a total of 8 total valence electrons.. Drawing the Lewis structure for CH 4 (named methane) requires only single bonds.It's one of the easier Lewis structures to draw. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell.
A step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use the periodic table to find the total number of vale... Lewis Structure of CH4. The lewis structure of carbon and hydrogen atom says- to form a single CH4 molecule, a total of eight valence electrons participate in the shared bonding to fulfill the need of eight more valence electrons. Here we will learn about how the lewis dot structure is drawn for CH4 molecule, step by step. Lewis Dot Structures 1. Methane - CH 4 Number of Valence Electrons: 4 from C and 1 each from 4 H = 8 Carbon is more electronegative than hydrogen, but hydrogen can never be the "central" atom, as it can only form 1 bond. Carbon always forms 4 bonds (2 electrons each). 2. Ammonia - NH 3 The calculation for methane shows that the carbon atom is associated with 8 electrons in the σ framework. This corresponds to four shape-determining electron ...
Dr. B. explains how to draw the Lewis dot structure for CH 4 (methane). The CH 4 Lewis Structure is one of the most frequently tested Lewis Structures.. Note that hydrogen atoms always go on the outside of a Lewis dot structure. This is because they can share a maximum of two electrons. Your Lewis Dot Structure for Methane should look like that of Figure 3.5.1 below. The structure of Figure 3.5.1 says that there are four atoms of Hydrogen, each involved in a covalent bond to one atom of Carbon. The octet rule has been satisfied for Carbon (there are eight electrons around it) so there is no net charge on the Methane molecule. ... Lewis Dot Structure for CH4 How to create a Lewis Dot Structure for CH4 # 2 Find the number of "octet" electrons for the molecule. C: 8 octet electrons x 1. How to draw the Lewis structure of methane, CH4 By José @ Periodic table with names diagramweb.net But seriously, you have an electron pair between the C and each of the H's in the ... Lewis symbols (also known as Lewis dot diagrams or electron dot diagrams) . Lewis dot dragram for methane: Methane, with molecular formula CH4, is shown. It is important to remember that Lewis valence dot diagrams are models that Methane is the main component of natural gas, and its chemical formula is CH4.
CH4 Lewis Structure, Hybridization, Molecular Geometry, Bond Angle and Shape. Methane is one of the simple organic molecules, given its straightforward structure. It has the chemical formula of CH4 and comprises one carbon atom forming bonds with four hydrogen atoms. The compound is one of the main constituents of natural gas.
What is the electron dot structure for CH4? The Lewis structure of the methane (CH4) molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each. Moreover, as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals, the hybridization of CH4 is sp3.
The chemical formula of methane is CH4. It is a gas that exists abundantly in nature. Now, let's know its structure: Lewis dot structure is a representation of ...1 answer · Top answer: Hint: To answer this question we should be aware of the chemical formula of methane, Lewis dot structure and the type of bond formed. This information will ...
The Lewis Dot Structure: The Lewis dot structure for any molecule can be found by following a general set of rules consisting of a few steps. These structures facilitate the proper description of ...
Lewis Structures. The Lewis structure, also known as the Lewis dot structure, is a representation of the arrangement of electrons which are shown as dots between atoms in a molecule.
Methane (CH4) lewis dot structure, molecular geometry, electron geometry, polar or nonpolar, bond angle Home > Chemistry Article > CH4 lewis structure and its molecular geometry Methane is a colorless and odorless gas formed from one atom of carbon and four atoms of hydrogen having the chemical formula CH4.
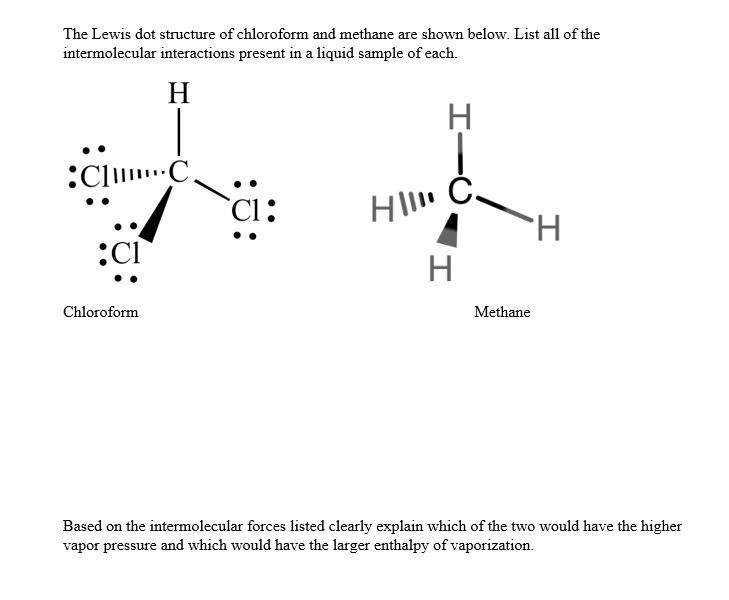
Drawing C is a Lewis electron dot structure for methane. Figure 1 Alternative Representations of Methane. Lewis Electron Dot Structures. Figure 2 animates the rules for drawing a Lewis electron dot structure using C 2 H 6 as an example. Click within the figure to view the animation.
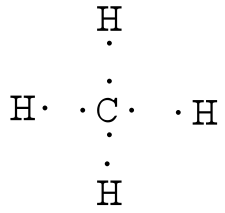
Answer: I would be lazy and look it up on the internet. But seriously, you have an electron pair between the C and each of the H's in the Lewis diagram a ala Why is that the correct diagram, you ask? First, each Hydrogen has only one electron to donate or share, and remember that Hydrogen's fil...
... atomic symbols and the shared pairs of electrons that constitute the four C-H covalent bonds. Drawing C is a Lewis electron dot structure for methane.Bond Line Notation: Molecular Formula
Answer: Well Carbon only has 4 valence electron, so it can bond at all four point. Hydrogen only has one valence electron and can only share one. Methane's (When it comes to hydrocarbons, "meth" stipulates one carbon, "ane" stipulates a single bond shared with hydrogens) molecular formula is CH4,...
May 18, 2020 — The Lewis Dot Structure for CH4 is shown above. These kinds of structures can also be shown by representing each of the bonds with two dots.
ISBN-13: 9780072534115 ISBN: 0072534117 Authors: Jeffrey A. Paradis, Jeffrey Paradis, Kristen Spotz Rent | Buy. Hands on Chemistry Laboratory Manual (1st Edition) Edit edition Solutions for Chapter 20 Problem 2: Draw the Lewis Dot Structure for methane, CH4.
Lewis Dot of Methane. CH 4. Back. 70 More Lewis Dot Structures. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule. The exception, of course, being the hydrogen's. They follow the duet rule (2 electrons). Methane is the first member of the alkane series.
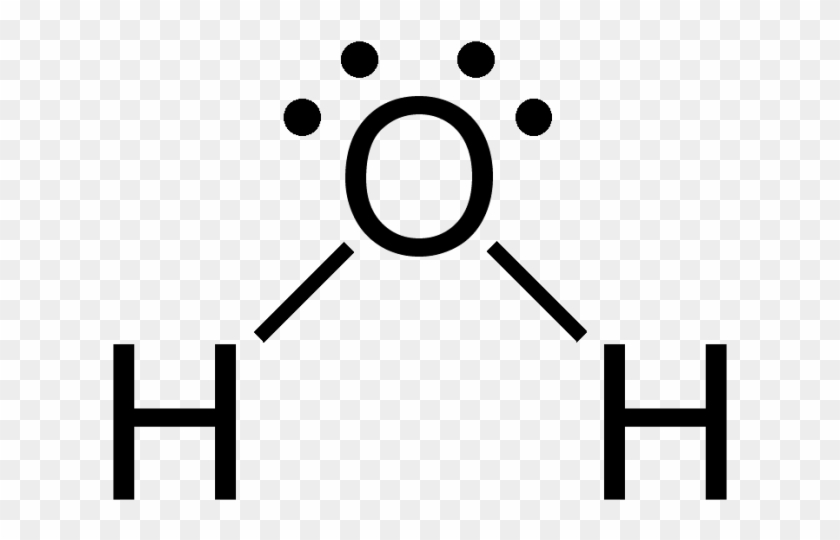
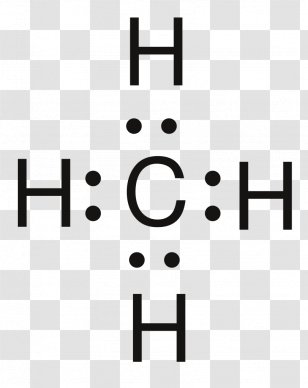
Lewis Structure of Methane (CH4)H|H--C--H|HorH..H : C : H..H. The Lewis Structure, or Lewis Dot Diagram, shows the bonding between atoms of a molecule and any electrons that may exist.
Lewis Dot Structure for CH4 #2 Find the number of "octet" electrons for the molecule. C: 8 octet electrons x 1 atom = 8 octet electrons H: 2 octet electrons x 4 . We show two ways to draw the CH4 Lewis structure, methane. We also have This info can then be used to determine the Lewis Dot Structure.
Lewis Dot Structure for CH4 (Methane) Properties of methane are described by Lewis Structure as cheaper natural gas than electricity. Methane, or CH4, is a natural gas that is relatively plentiful on earth, making it an environmentally effective source.
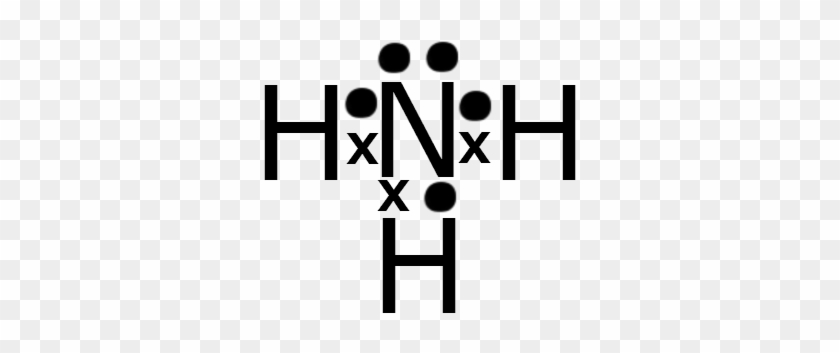
Lewis Dot Structures can be produced by following a sequence of steps. Let's produce a Lewis Dot Structure for: NH 4 + (the ammonium ion). Step 1: Count valence electrons: N = 5 4 x H = 4 x 1 = 4 "+" = -1 Total = 5+4-1= 8 electrons = 4 bonds and lone pairs. Step 2:!Arrange the atoms (identify a central atom, if possible).
CH4 Lewis Structure. Answer: CH4 Lewis structure (Methane electron dot structure) is that type of diagram where we show the total 8 valence electrons of CH4 as dots , or dots and dashes (-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash (-) or dots ( ) but a lone pair of two electrons is shown by dots [ ].
















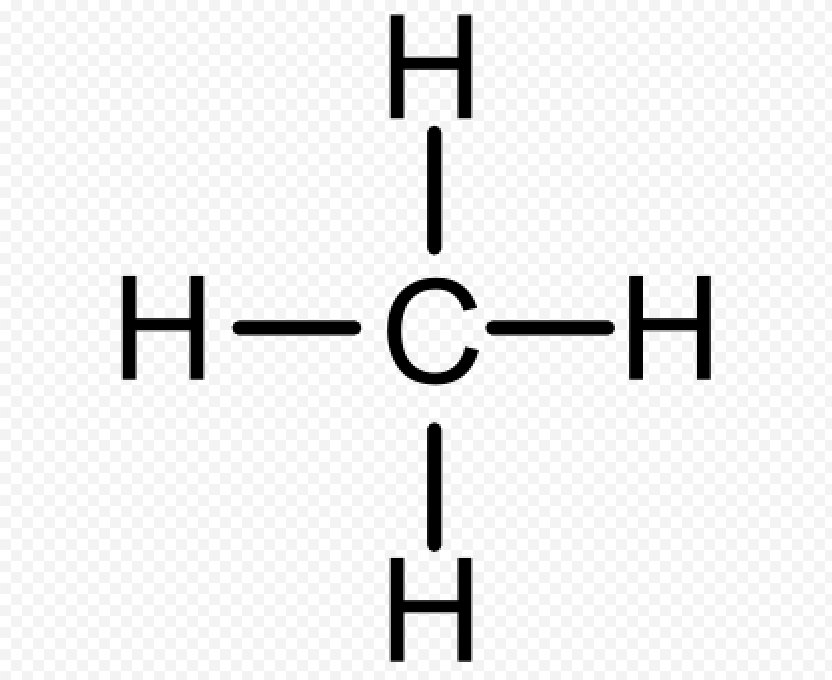






0 Response to "41 lewis dot diagram for methane"
Post a Comment