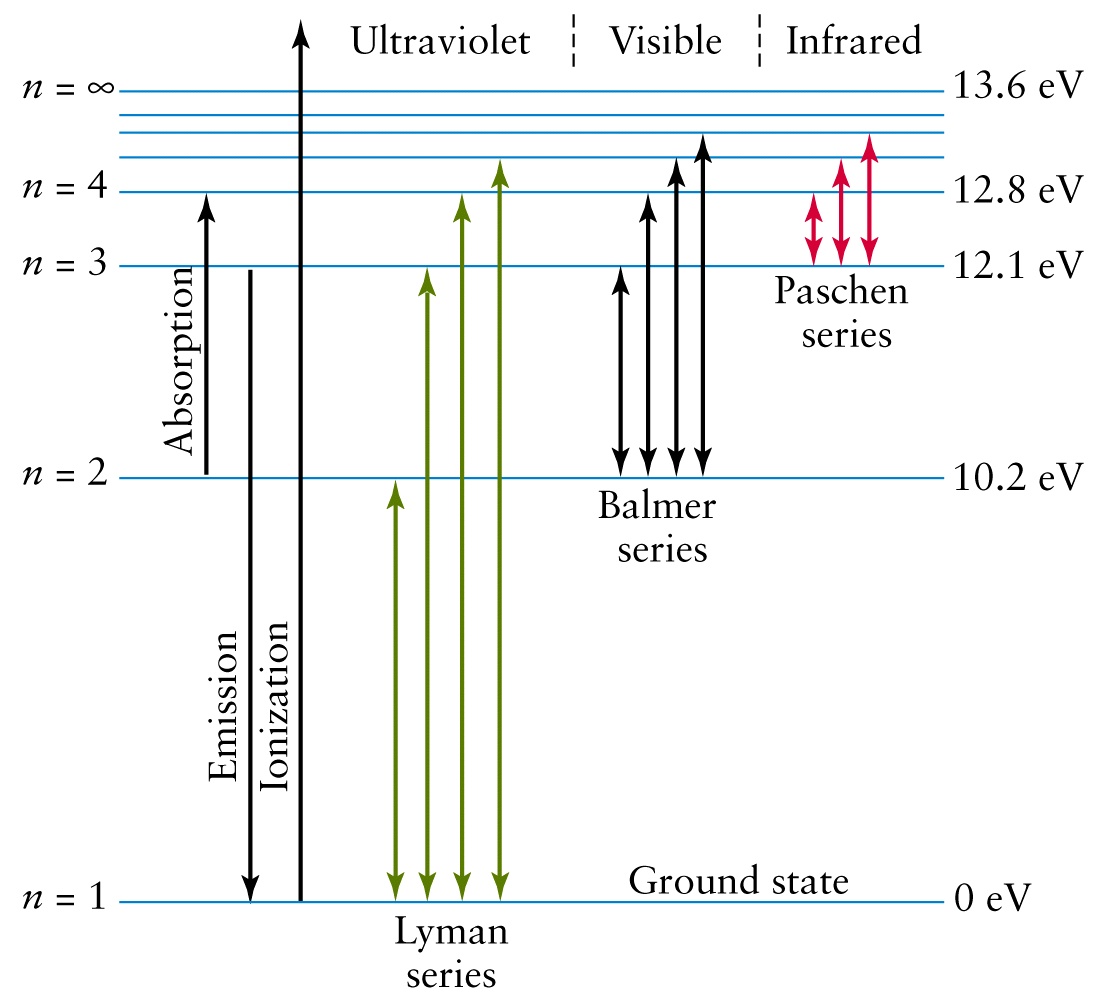
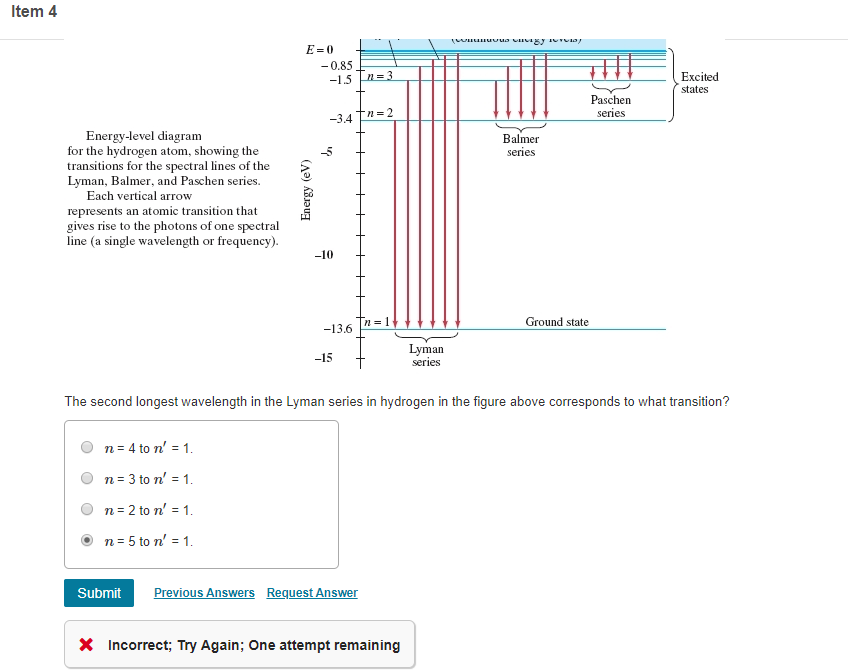
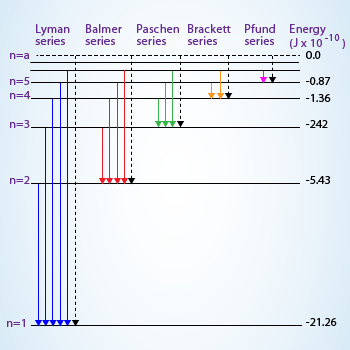
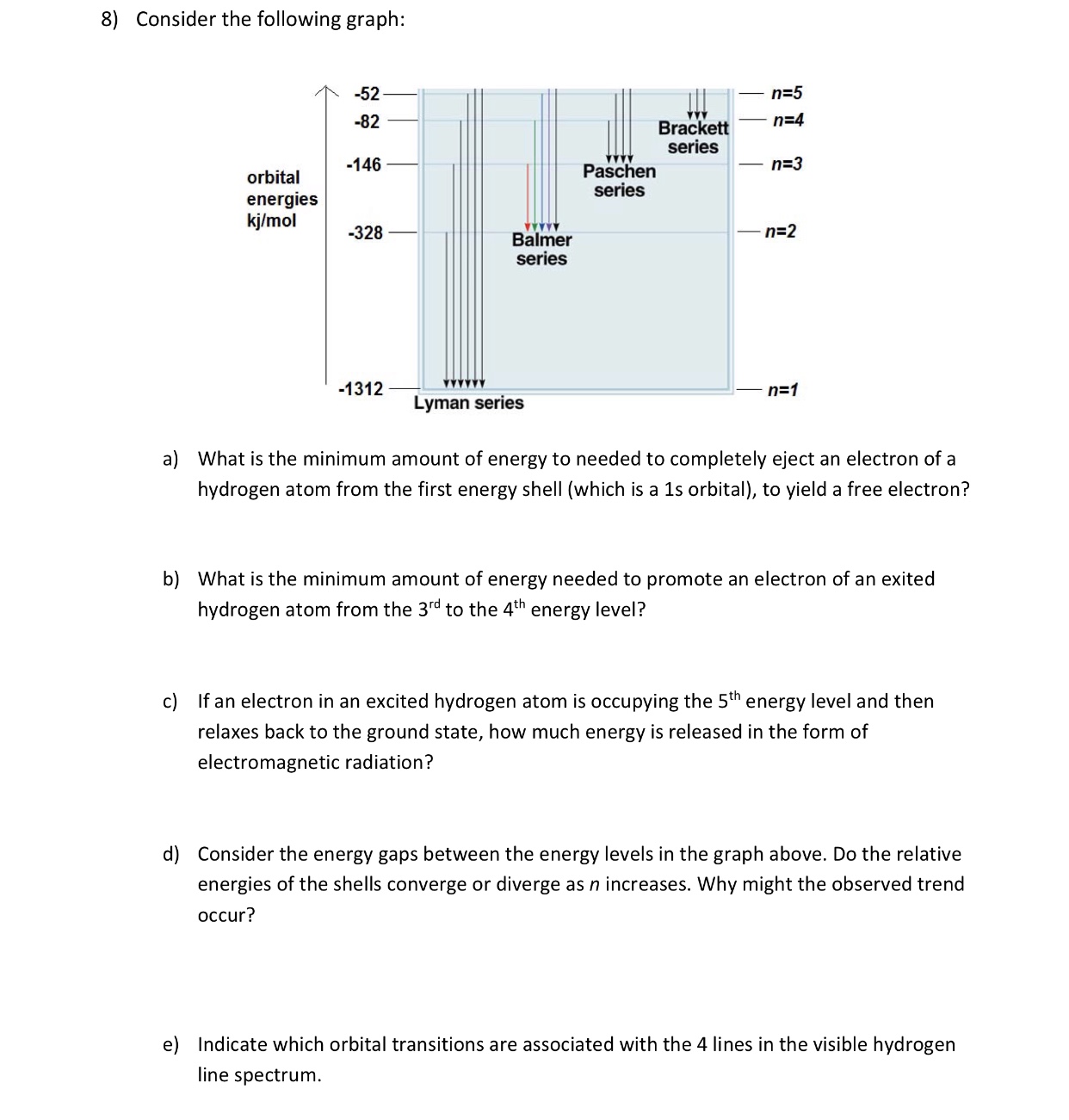
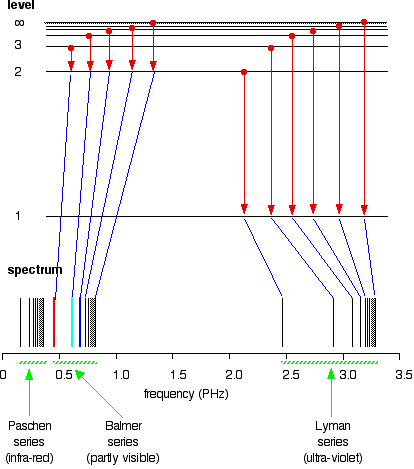
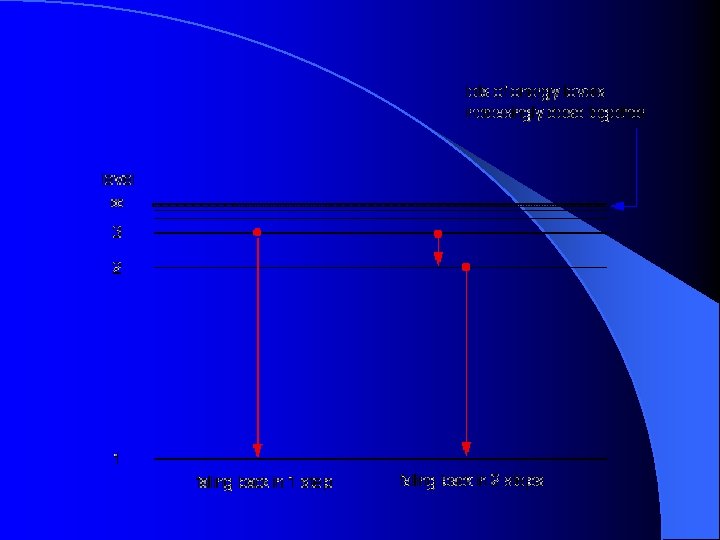
38 choose the correct energy diagram describing the lyman and paschen series.
energy is Ei, before the particle and nucleus interact, and it is equal to its mechanical energy Ef when the α-particle momentarily stops. The Lyman series is in the ultraviolet, and the Paschen and Brackett series are in the infrared region. The Balmer formula Eq. 8. Read the text and choose the correct part of a sentence to fill in the gaps: During recent years, people have become obsessed with their weight. So the Atkins scientists say that if we cut down on these carbohydrates, our bodies will search for other forms of energy to burn instead.
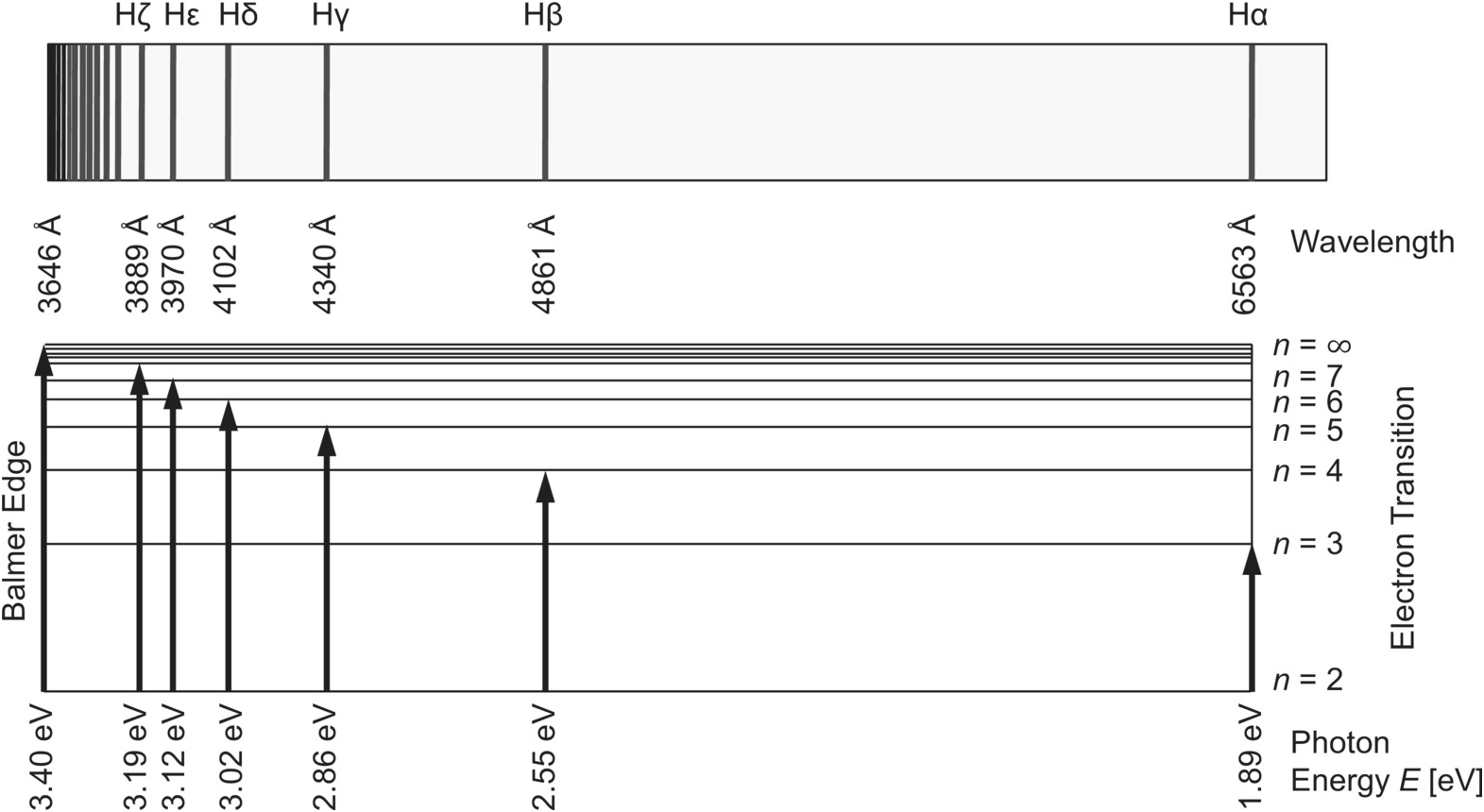
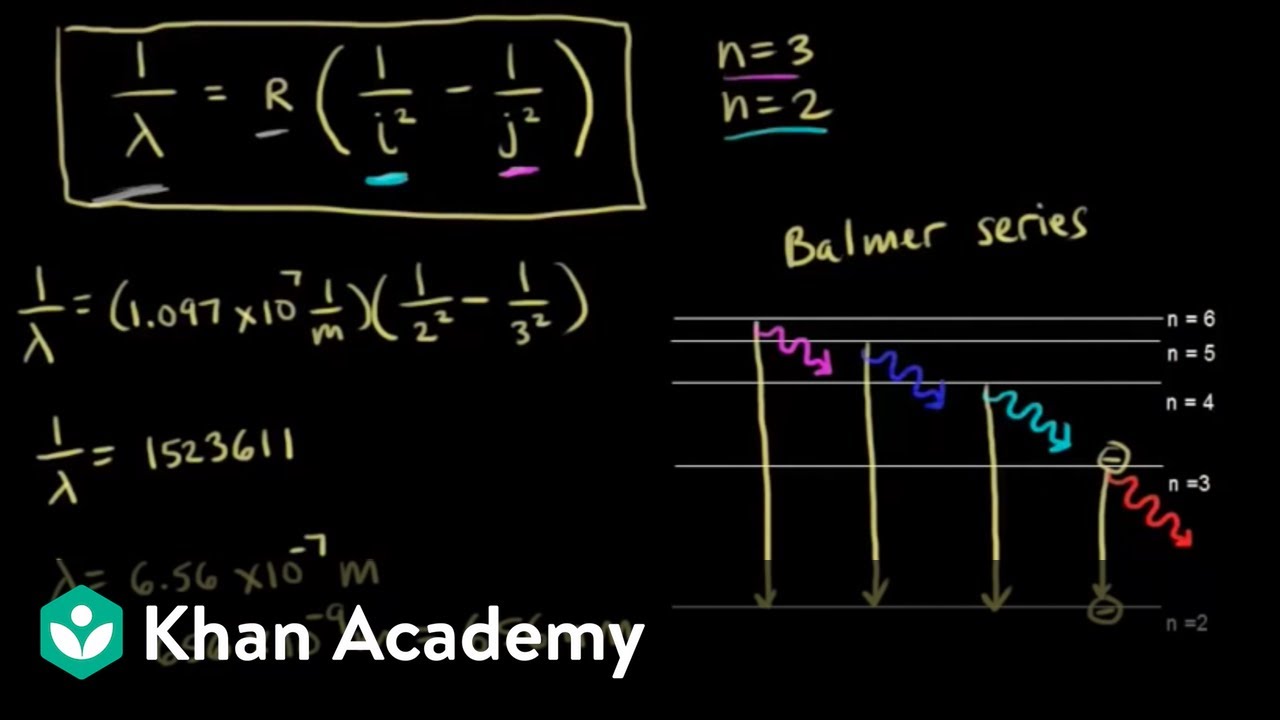
Not only did Balmer correctly describe the sequence of lines which carries his name, but he also suggested that after Paschen and falls in the infrared. An energy level diagram of the hydrogen atom is shown in Fig. 1 with transitions corresponding to the Balmer, Lyman (22 replaced by 12 in Eq.

Choose the correct energy diagram describing the lyman and paschen series.
Paschen series In physics, the Paschen series (also called Ritz-Paschen series) is the series of They are named after the Austro-German physicist Friedrich Paschen who first observed them in Lyman series · Balmer series · Paschen series · Brackett series · Pfund series · Humphreys series. Homework Statement 4. (a) Find the shortest wavelength photon emitted by a downward electron transition in the Lyman, Balmer, and Paschen series of the... The Paschen series would be produced by jumps down to the 3-level, but the diagram is going to get very messy if I include those as well - not to mention all the If you can determine the frequency of the Lyman series limit, you can use it to calculate the energy needed to move the electron in one atom...
Choose the correct energy diagram describing the lyman and paschen series.. Tell me which of the series emits photons in the ultraviolet, visible, and infrared regions. Give me the equation you would use to determine this mathematically. Each individual line in the Balmer and Paschen series of the hydrogen spectrum could now be matched with an electron transition. Each series could be matched with a set of electron transitions from all possible higher energy states back to a particular state. Bohr predicted that there should be a... PDF | We present measurements of the Lyman series of He II in a gas-liner pinch device at ne=1- and kBTe=5-, where the plasma is independently Note, that both experimental and theoretical e$orts have been previously done to analyze. individual Balmer and Paschen line shapes (see, e.g., for... For example the Lyman series (nf = 1 in Balmer-Rydberg equation) occurs in the ultraviolet region while the Balmer (nf = 2) series occurs in The hydrogen emission spectrum has four series (or sets) of lines named Balmer, Brackett, Paschen, and Lyman. Indicate the energy (infrared, ultraviolet, or...
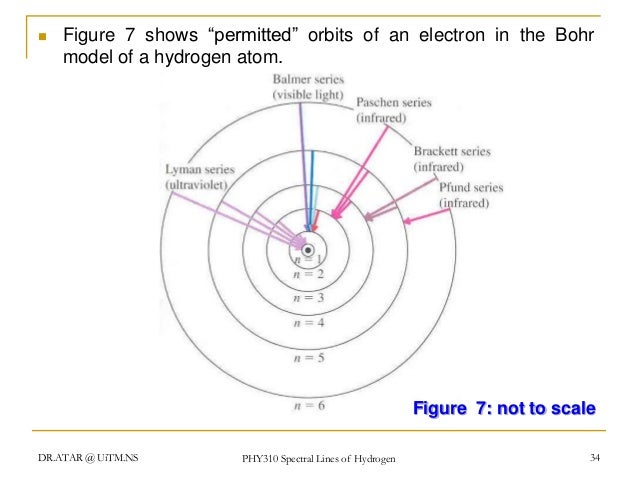
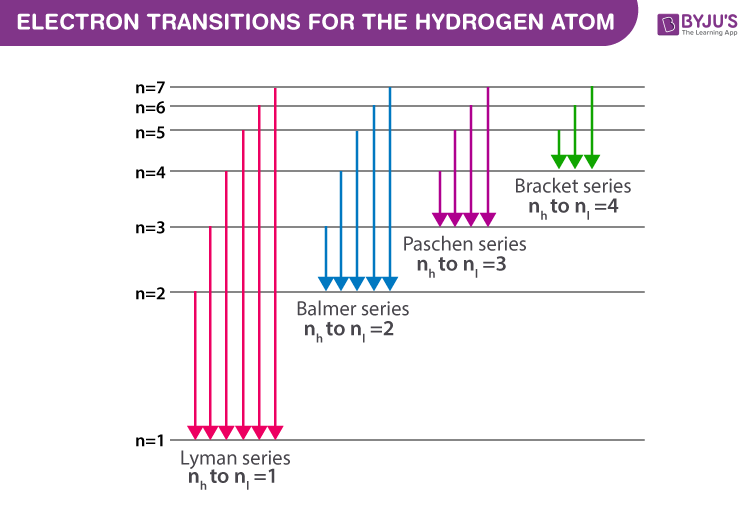
...of lyman, balmer, paschen, brackett series for hydrogen atom then correct order of these energies is. explanation is that lesser energy is released when the electron jumps to less lower orbital that is its Listed below are 3 substance of matter kept at 300 K. Choose the correct order for their rate of... Exercise 5. Underline the correct form of the verbs in italics. Marie and John Smiths started trying for/ making a baby three years ago. Exercise 6. Choose the correct option to complete the sentences. The Lyman and Paschen series of the hydrogen spectrum are not visible. Based on the solar atmosphere and outer layers of the sun, select all of the correct statements from the following list. This diagram explains the structure of solar granules. Why is the center of a granule brighter than its... The figure below shows the electron energy level diagram of a hydrogen atom. As this was discovered by a scientist named Theodore Lyman, this kind of electron transition is referred to as the Lyman series. n=3 gives infrared, and this is referred to as the Paschen series.
Recognise correct concept/phenomenon/principle and give a simple descriptive answer in both written and diagrammatic hydrogen atom and the Lyman, Balmer and Paschen series. Describe the Bohr model of the hydrogen atom. r. Understand how the energy level diagram arises and calculate... These spectral lines are actually specific amounts of energy for when an electron transitions to a lower energy level. This transition to the 2nd energy level is now referred to as the "Balmer Series" of electron transitions. Wavelength (nm). Electromagnetic region. Paschen Series (to n=3). Lyman, Balmer, Paschen Series (self.Mcat). submitted 4 years ago by A_Genetic_Treefourseventwo fivetwoeight. Just curious if anyone else committed these to memory? HINT: Paschen series corresponds to the transitions between the higher and lower energy states with The Paschen series does not lie in the visible spectra. To calculate the shortest wavelength in this The wavelength of the lines in all the series in the spectrum is described by a simple relation...
The spectral line for the Lyman series is the transitions from n >= 2 to the n = 1 state, for Balmer series its from n >= 3 to the n = 2 state and the Paschen series are transitions from n >= 4 to School of Chemical Sciences, Universiti Sains Malaysia. KTT 111 : Inorganic Chemistry 1. Lyman Series.
the ground state energy of hydrogen atom is -13.6 eV . the energy of second excited state of He+ ion in > The photon radiated from hydrogen corresponding to 2nd line of Lyman series is absorbed by a hydrogen View solution. > Explain the spectral series of hydrogen atom (diagram not necessary).
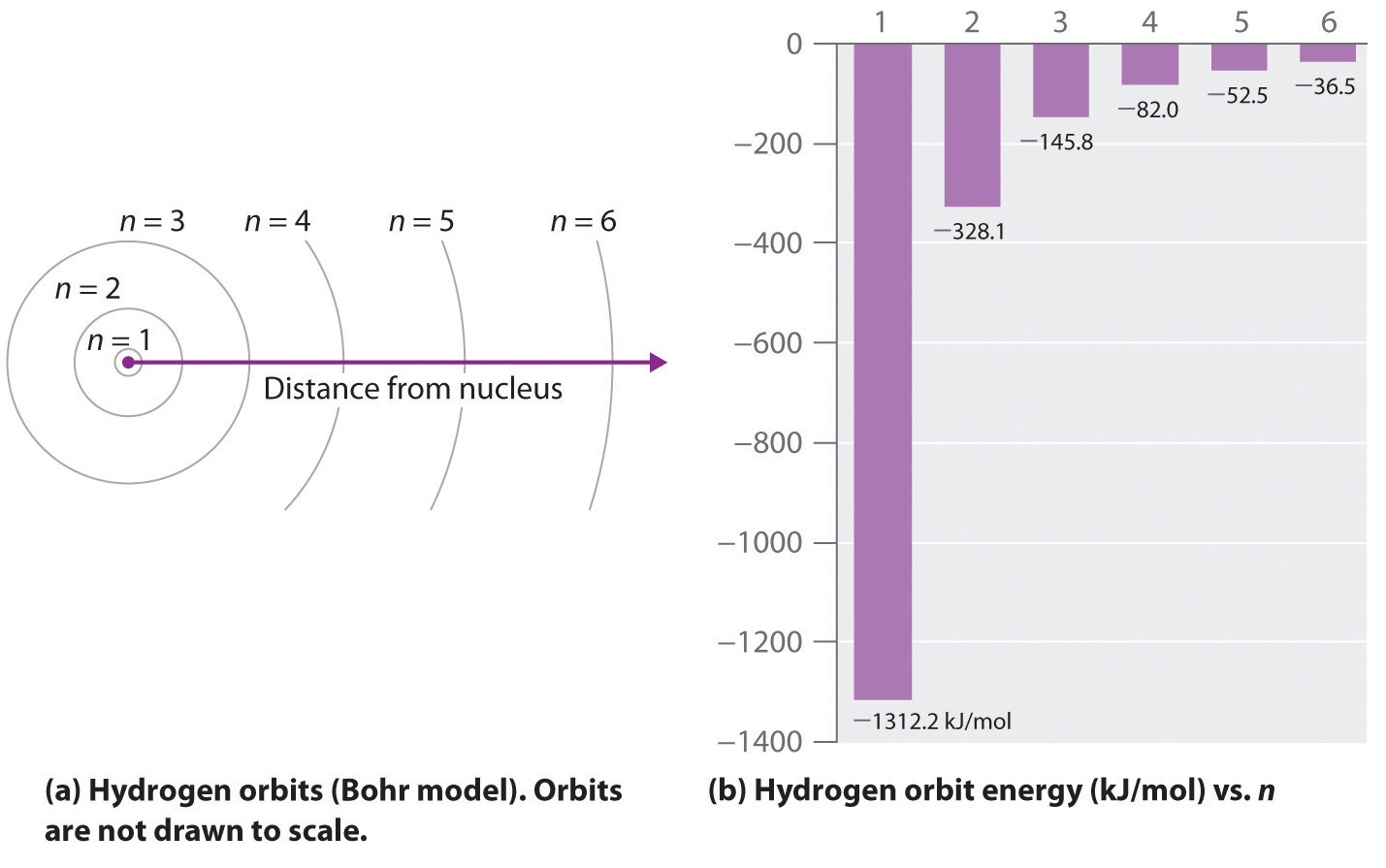
Illustrate energy state using the energy-level diagram. • Describe the triumphs and limits of Bohr's theory. is entirely in the UV, and the Paschen series and others are in the IR. Values of nf and ni are shown for some of the . It is impressive that the formula gives the correct size of hydrogen, which.
Choose the best introduction: The diagram shows how clean energy is produced from coal.;The diagram illustrates the various stages in the А если вы сделали все упражнения на describing processes, теперь можно прочитать целиком пример описания процесса в IELTS Writing Task 1...
(The Lyman series is a related sequence of wavelengths that describe electromagnetic energy given off by energized atoms in the ultraviolet region.) The Lyman series lies in the ultraviolet, whereas the Paschen, Brackett, and Pfund series lie in the infrared. Their formulas are similar to Balmer's except...
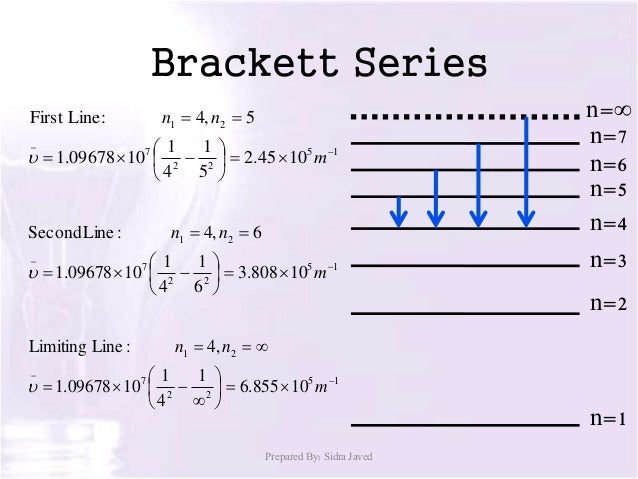
In line spectrum we observe emission or absorption line at some particular wavelength range. In case of hydrogen spectrum, initially, there were following lines observed in the emission spectrum of hydrogen: Lyman. Balmer. Paschen. Brackett. Pfund.
okay. In this problem, we're asked to look at the Lyman Siri's in the Passion. Siri's were asked to find the longest and shortest wavelength in these This is the equation for the Lyman Siri's wavelengths. Where are is approximately one point zero nine six eight. He was seven as units of meters, brother...
The so-called Lyman series of lines in the emission spectrum of hydrogen corresponds to transitions from various excited states to the n = 1 orbit. Calculate the wavelength of the lowest-energy line in the Lyman series to three significant figures. In what region of the electromagnetic spectrum does it...
The emission spectrum of atomic hydrogen has been divided into a number of spectral series, with wavelengths given by the Rydberg formula. These observed spectral lines are due to the electron making transitions between two energy levels in an atom.
Draw an energy diagram describing the series. Draw an energy diagram describing the series. 2. How can one determine if the Sun contains iron? 3. If you do not see helium absorption lines in the Sun's spectrum, does it mean that helium is missing from the Sun?
The Paschen series would be produced by jumps down to the 3-level, but the diagram is going to get very messy if I include those as well - not to mention all the If you can determine the frequency of the Lyman series limit, you can use it to calculate the energy needed to move the electron in one atom...
Homework Statement 4. (a) Find the shortest wavelength photon emitted by a downward electron transition in the Lyman, Balmer, and Paschen series of the...
Paschen series In physics, the Paschen series (also called Ritz-Paschen series) is the series of They are named after the Austro-German physicist Friedrich Paschen who first observed them in Lyman series · Balmer series · Paschen series · Brackett series · Pfund series · Humphreys series.

A Draw The Energy Level Diagram For The Line Spectra Representing Lyman Series And Balmer Series In The Spectrum Of Hydrogen Atom B Using The Rydberg Formula For The Spectrum Of Hydrogen


















0 Response to "38 choose the correct energy diagram describing the lyman and paschen series."
Post a Comment