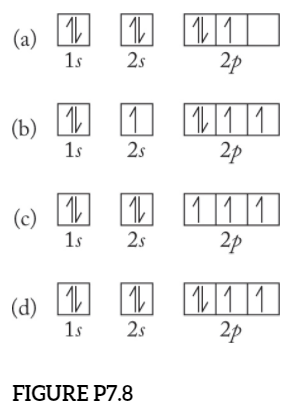
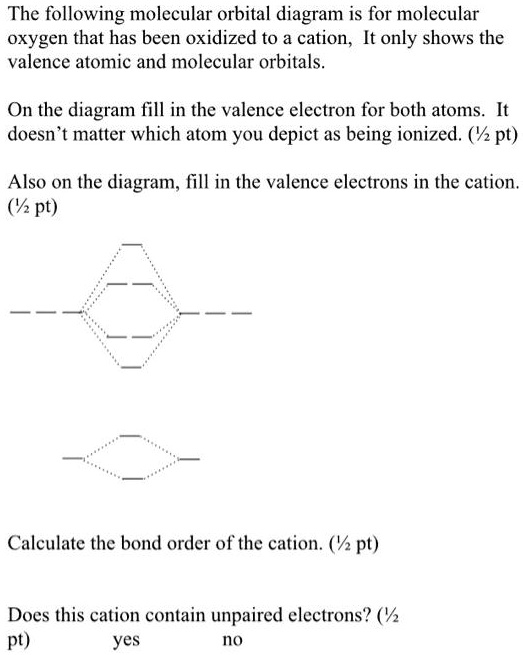
38 orbital diagram for oxygen cation
I have to brain storm some ideas for a research project in my biomaterials class. Would it be possible to make a composite out of SiC and Ti 6AI-4V? how to draw a lewis structure for Ti-6AI-4V? Ti 6AI-4V is made up of 6% aluminium, 4% vanadium, 0.25% (maximum) iron, 0.2% (maximum) oxygen, and the remainder titanium. I don't believe the percentages will play a part in a Lewis structure type diagram but just incase. At first I thought it was Ti and 6 groups of AI but then I remembered that I isn't... MO Diagram of SF2. MO diagrams are a good way to represent the different properties of a compound. These properties include shape, bond energy, bond angle, and more such things. With the help of this diagram, we can showcase the energy that different energy orbital acquires and have.
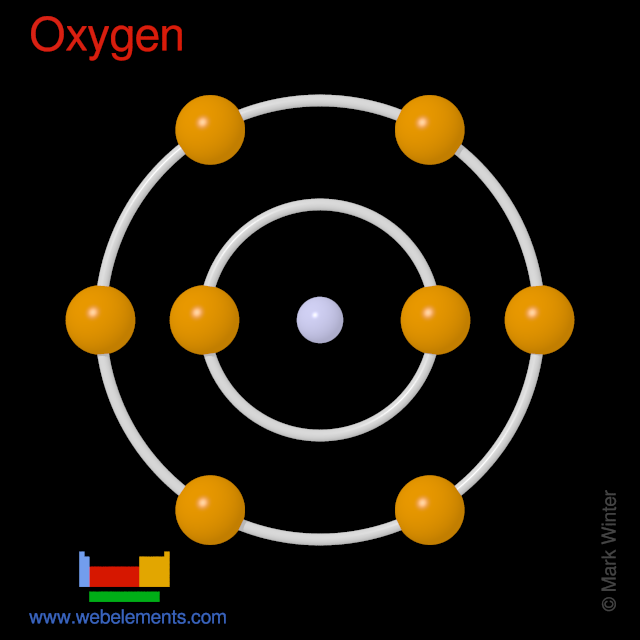
Electronic configuration of Oxygen atom is. Oxygen-15 is synthesized through deuteron bombardment of nitrogen-14 using a cyclotron. Elements with the same electrons or electron configurations are said to be isoelectronic. Draw The Atomic Structure Of Oxygen Ion O 2 Brainly In . Oxygen Electron Configuration O With Orbital Diagram

Orbital diagram for oxygen cation
Nov 03, 2021 · Fig. 1: Structure an doping of the prototype system. Fig. 2: The experimental phase diagram of the AMn 7 O 12 series, plotted as a function of formal B-site valence, constructed from 21 samples ... A molecule is an electrically neutral group of two or more atoms held together by chemical bonds. Molecules are distinguished from ions by their lack of electrical charge.. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and molecule is often used when referring to polyatomic ions. For example, we know that Oxygen always forms 2- ions when it makes an ion. ... Orbital Diagrams. Another way to represent the order of fill for an atom is by using an orbital diagram often referred to as "the little boxes": The boxes are used to represent the orbitals and to show the electrons placed in them. The order of fill is the same but ...
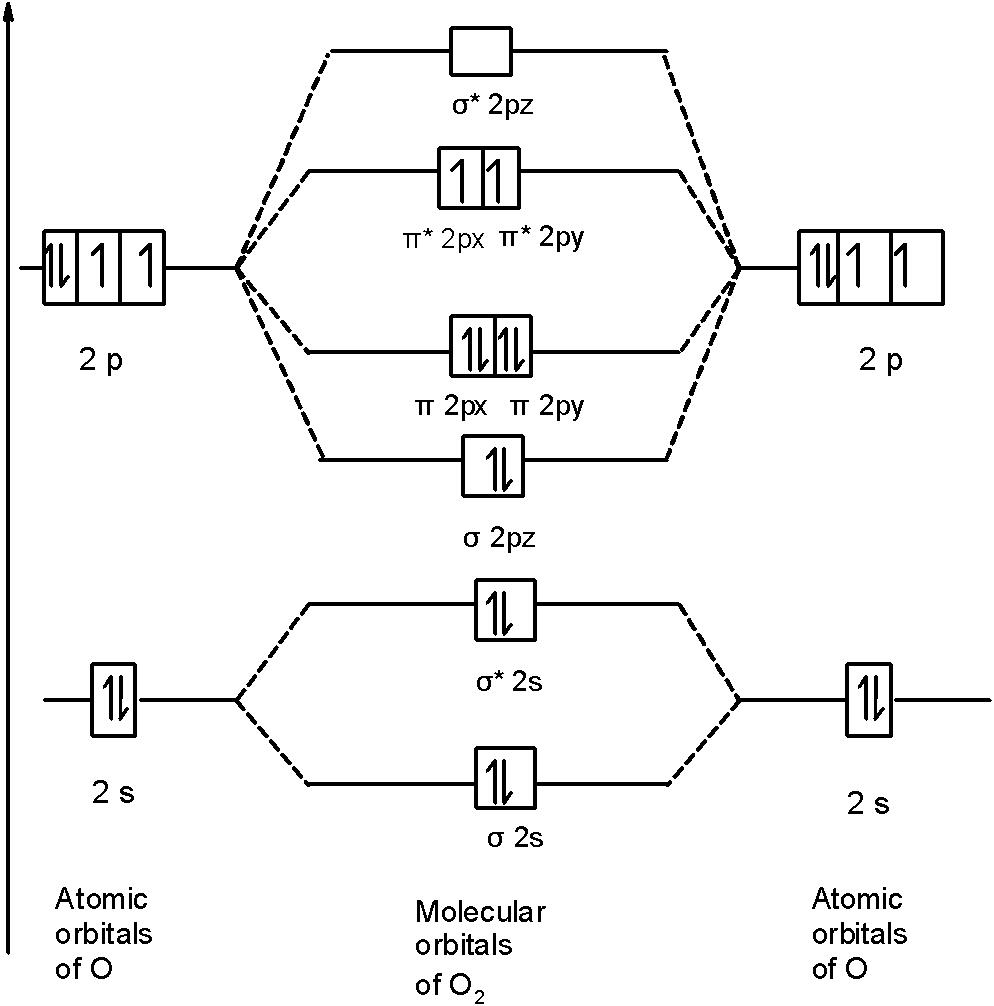
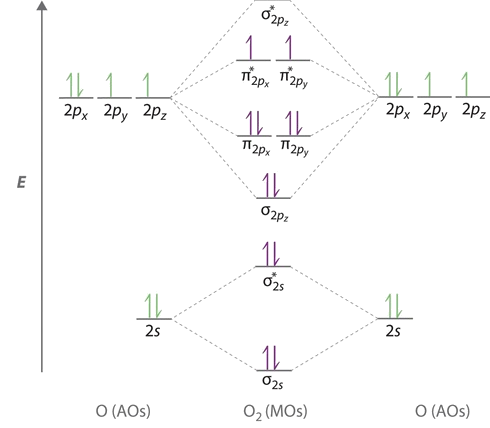
Orbital diagram for oxygen cation. We would like to show you a description here but the site won’t allow us. The bond length in the oxygen species can be explained by the positions of the electrons in molecular orbital theory. To obtain the molecular orbital energy-level diagram for O 2, we need to place 12 valence electrons (6 from each O atom) in the energy-level diagram shown in Figure 9.10.1 . We again fill the orbitals according to Hund's rules ... b. Based on its position in the periodic table, explain how you know that your answer to part a is the correct number of electrons for oxygen. The atomic number of oxygen is 8. 8. Examine the orbital diagrams and electron configurations in Model 2. Using the following elec- tron configuration: a. Underline the energy levels. b. Circle the. The reason for the polarity also emerges due to the presence of lone pair on the oxygen atom in the H3O+ molecule. The net dipole comes out to be some non-zero value that makes H3O+ a polar molecule. H3O+ Molecular Orbital (MO) Diagram. A molecular orbital diagram of any molecule gives us an idea about the mixing of orbitals in the molecule.
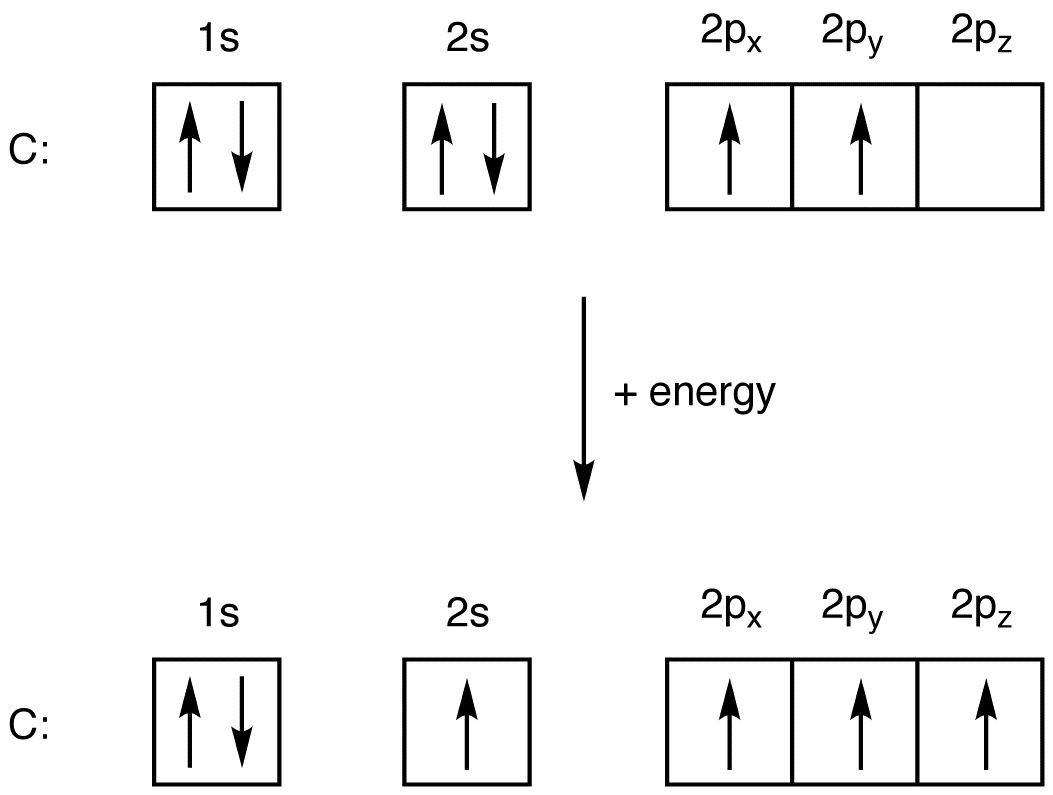
Answer (1 of 3): I modified the picture from this post: What's the MOT diagram of O2 +2 ion? and modified it to be O2 2+ (since sadly enough I am about as advanced with artistic programs on pc as a rock). How you basically do these questions is by first drawing the empty AO and MO, then counting ... The molecular orbital diagrams for molecules and ions are drawn from the order of increasing energies shown in the molecular orbital configuration. Always remember that the number of molecular orbitals formed must be equal to the number of atomic orbitals that were combined in the molecule. Box spin diagram of outer electron orbitals for the electron configuration of the 15 Phosphorus P 1s22s22p63s23p3 Ne3s 3p P pblock Gp5 Build the orbital diagram for the ion most likely formed by phosphorus. The rules for orbital filling diagrams. Energy 0 1 1 x 5. Now this is only one way we can draw the electron dot diagram for Oxygen. 2p. To figure out the configuration on your own, you can follow the orbital diagram to map out which shells will be filled first. According to Hund’s rule, electrons fill all orbitals of equal energy with one electron before pairing electrons. That means that for carbon, the two electrons in the 2p subshell would not occupy the same orbital.
ClO3- Lewis Structure, Molecular Geometry, Hybridization & Shape. The chemical formula ClO 3- represents Chlorate ion. Chlorine can reach oxidation states of +1, +3, +5 and +7. In this case, as seen in the figure, Chlorates exist in a +5 oxidation state. With an abundance of oxidizing elements, the Chlorate ion and its salts make for powerful ... The valence molecular orbitals in both atoms are the 2 s and 2 p orbitals. The molecular orbital diagram for carbon monoxide (Figure 5.3.1. 1) is constructed similarly to how you would construct dicarbon or dioxygen, except that the oxygen orbitals have a lower potential energy than analogous carbon orbitals. The labeling of molecular orbitals ... Salts of cycloheptatrienyl cation (tropylium ion) are stable in water solution, again reflecting the stability of this 6 π-electron cation. Antiaromaticity Conjugated ring systems having 4n π-electrons (e.g. 4, 8, 12 etc. electrons) not only fail to show any aromatic properties, but appear to be less stable and more reactive than expected. In the diagram, the left-hand side consists of the atomic orbitals of carbon. Likewise, the left side has AO's of oxygen. And in the middle is the MO. We can see that the 2s orbital of oxygen is not involved in mixing and remains a nonbonding orbital. The reason for this is the high energy difference between the orbitals of carbon and the 2s ...
Molecular Orbital Diagrams. This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H 2 +. Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as is the case for atomic ...
2.3: Molecular orbital theory- conjugation and aromaticity. Valence bond theory does a remarkably good job at explaining the bonding geometry of many of the functional groups in organic compounds. There are some areas, however, where the valence bond theory falls short. It fails to adequately account, for example, for some interesting ...
The Jahn–Teller effect (JT effect or JTE) is an important mechanism of spontaneous symmetry breaking in molecular and solid-state systems which has far-reaching consequences in different fields, and is responsible for a variety of phenomena in spectroscopy, stereochemistry, crystal chemistry, molecular and solid-state physics, and materials science.
The molecular energy level diagram for \(\ce{HCl}\) is reproduced in Figure 10.4.5 Figure 10.4.5 : Molecular Orbital Energy-Level Diagram for HCl. The hydrogen 1s atomic orbital interacts molecular orbitals strongly with the 3p z orbital on chlorine, producing a bonding/antibonding pair of molecular orbitals. The other electrons on Cl are best ...
To determine the bond order between the carbon and oxygen atom, notice it is a double bond. ... For the bond order formula of simple molecules and ions, draw the molecular orbital diagram and ...
a) Crystal structure of Co 2 YZ Heusler compounds. The magnetic moment M of Co 2 YZ can be calculated according to the Slater-Pauling rule: M=(N v-24) μ B.b) Illustration of molecular orbital diagram of Co 2 MnAl. c) The magnetic moment and electron occupation of the six selected Co 2 YZ compounds. For simplicity, only the part (light red region) which presents different electron occupation ...
For example, we understand that Oxygen always forms 2- ions when it renders an ion. This would include 2 electron to its typical configuration make the brand-new configuration: O2- 1s22s22p6 . With 10 electron you must note the oxygen"s electron construction is now exactly the exact same as Neon"s.
There are 118 elements in the periodic table. Column 6 Draw the electron dot diagram for the anion. Oxygen will gain 2 electrons. A cation is specific to metals and obtains a positive charge through the loss of electrons that throws off the balance leaving more protons than electrons. Cations and Anions form from Neutral Atoms.
Step 4. Search for the bond forming between the magnesium and oxygen atoms: The ionic bond will be formed between the atoms as magnesium will be donating two of its valence electrons from the 3s shell to fulfill the electron deficiency in the oxygen atom. Step 5. Now draw the Lewis structure of magnesium oxide (MgO): From the diagram, it is ...
The remaining four electrons will go in the 2p orbital. Therefore the O electron configuration will be 1s22s22p4. Video: Oxygen Electron Configuration Notation ...24 Oct 2016 · Uploaded by Wayne Breslyn
same family. The orbital diagram will show one less e- for each halogen family and each noble gas family with full valence electron shell. A full shell is what the elements strive for so noble gas members require larger IE in order to lose their e-. 7. Where would the largest jump in ionization energies be for oxygen? (with the
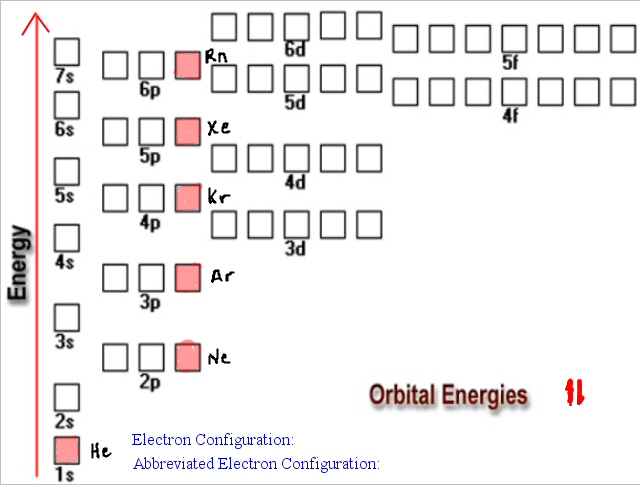
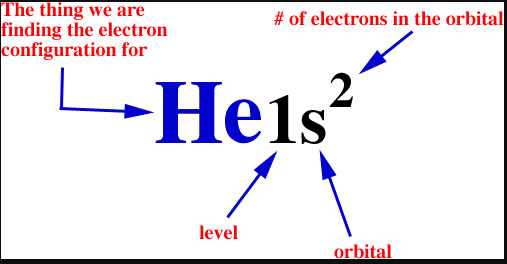
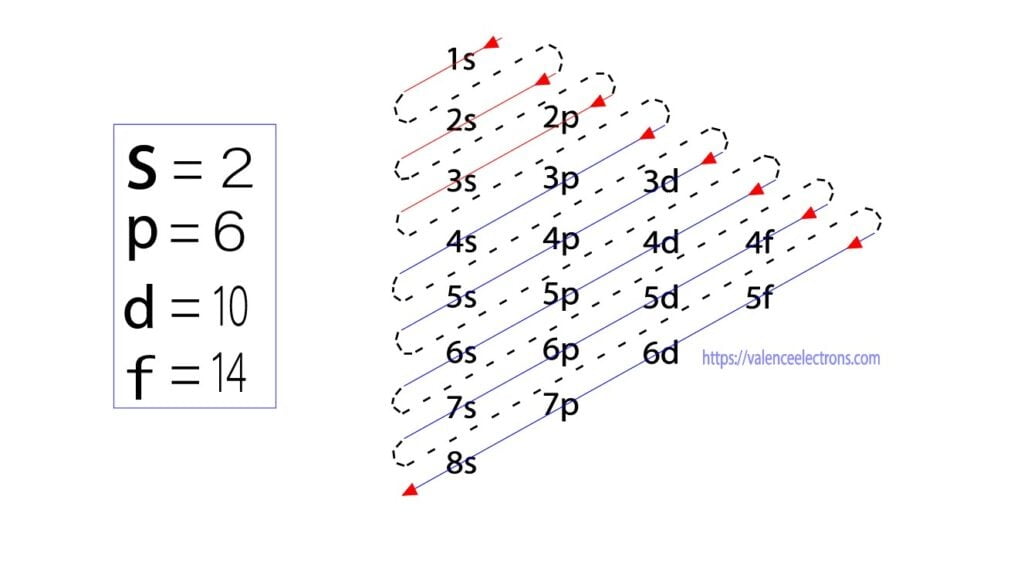
There are four different orbital shapes: s, p, d, and f. Within each shell, the s subshell is at a lower energy than the p. An orbital diagram is used to determine an atom's electron configuration. There are guidelines for determining the electron configuration of an atom. An electron will move to the orbital with lowest energy.
A molecular orbital diagram of any type of compound provides us an idea about the bonding that the orbitals. It likewise helps united state to uncover the link order, link length, bond stamin of the molecule. In the diagram, the left-hand side is composed of the atom orbitals the carbon. Likewise, the left side has actually AO's the oxygen.
Molecular orbital diagram for o2- ion. Molecular orbital diagram for o2 2. This ion has been observed in the gas phase. O2 molecular orbital diagram oxygen has a similar setup to h 2 but now we consider 2s and 2p orbital s. This diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of a molecular orbital ...
Hence, three sp2 hybrid orbitals are formed whereas the 2pz orbital remains unchanged and hence, 2pz orbital is used for the formation of a pi bond with the oxygen atom. The sideways overlapping of atomic orbitals results in the formation of a pi bond. The orbital diagram of nitryl fluoride, representing only the sigma bonds, is shown below.
15 Sept 2016 — The electron configuration for oxygen is: 1s^2 2s^2 2p^4 This video will walk you through the step of writing orbital diagram.1 answer · http://www.thestudentroom.co.uk/showthread.php?t=3933809&page=30 Explanation: The electron configuration for oxygen is: 1s22s22p4 This video will ...
Stable ion oxygen sr strontium br bromine zn zinc cs cesium Making it to be 10 total electrons from the aluminum ion, so therefore the answer would be: This means that the electron configuration of the li^(+) cation will be li^(+): Predict the charge of the most stable ion this atom forms. Groups 1 and 2 are the s block.
For example, we know that Oxygen always forms 2- ions when it makes an ion. ... Orbital Diagrams. Another way to represent the order of fill for an atom is by using an orbital diagram often referred to as "the little boxes": The boxes are used to represent the orbitals and to show the electrons placed in them. The order of fill is the same but ...
An orbital diagram helps to determine the electron configuration of an element. Electrons that occur together in an orbital are called an electron pair. The state in which all electrons have the lowest possible energy. Draw the mo diagram for molecular oxygen, o2. The orbital diagram is also one way of representing the electron configuration.
Definition of atomic orbital diagram for oxygen: An orbital is the region of space around the nucleus within which the probability finding an electron of given energy is maximum. The diagram of this region gives the diagram of the orbital. The plot of angular wave function or square of angular wave functions give us the diagram of orbitals.
Exercise 221 Draw an orbital diagram for nitrogen Z 7. In order to write the C electron configuration we first need to know t. Therefore the C electron configuration will be 1s22s22p2. Since 1s can only hold two electrons the next 2 electrons for C goes in the 2s orbital. 2 See answers. On the removal of 2 valence electrons it will be become ...
For example, we know that Oxygen always forms 2- ions when it makes an ion. ... Orbital Diagrams. Another way to represent the order of fill for an atom is by using an orbital diagram often referred to as "the little boxes": The boxes are used to represent the orbitals and to show the electrons placed in them. The order of fill is the same but ...
A molecule is an electrically neutral group of two or more atoms held together by chemical bonds. Molecules are distinguished from ions by their lack of electrical charge.. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and molecule is often used when referring to polyatomic ions.
Nov 03, 2021 · Fig. 1: Structure an doping of the prototype system. Fig. 2: The experimental phase diagram of the AMn 7 O 12 series, plotted as a function of formal B-site valence, constructed from 21 samples ...











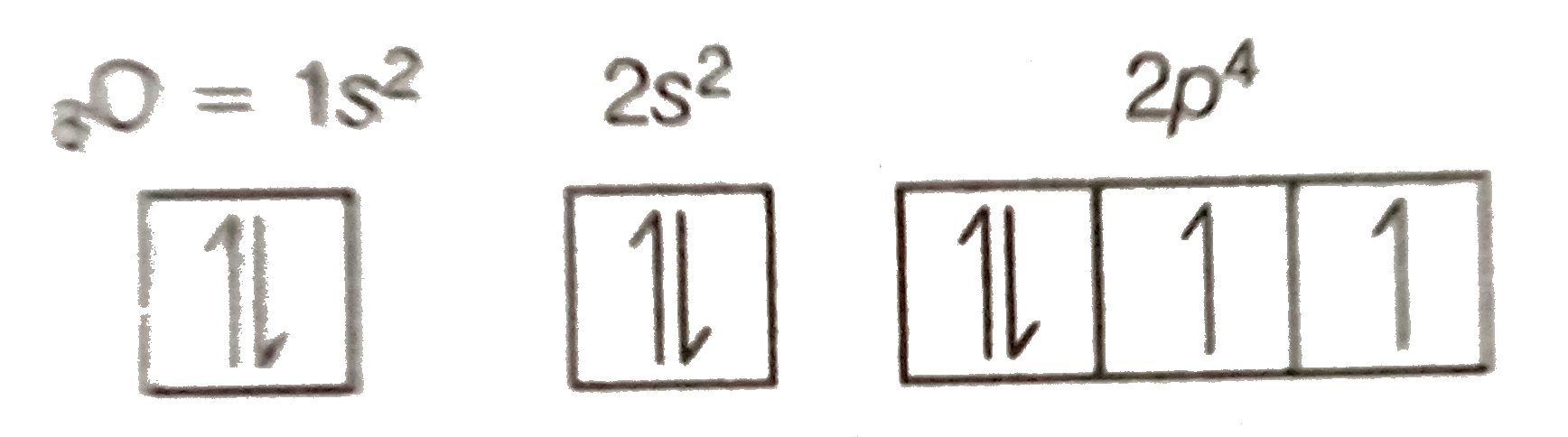





0 Response to "38 orbital diagram for oxygen cation"
Post a Comment