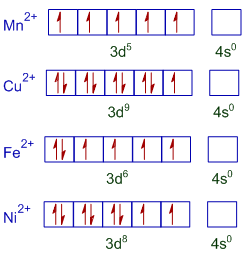
39 fe2+ orbital diagram
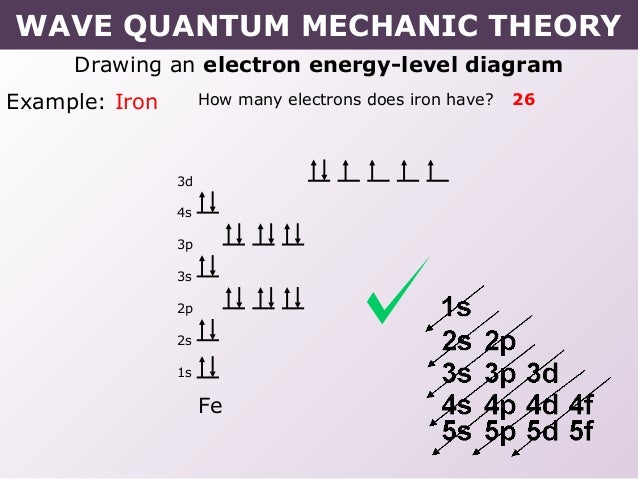
18 Nov 2017 — The outermost shell, in this case, is the 4s orbital. So removing 2 electrons would leave you with the electron configuration of [Ar] 3d^6.Electron Configuration of Fe2+5 Nov 2013Electron Configuration for Fe2+14 Sept 2016More results from lavelle.chem.ucla.edu 28 Aug 2020 — Use an orbital diagram to describe the electron configuration of the valence shell of each of the following atoms:.
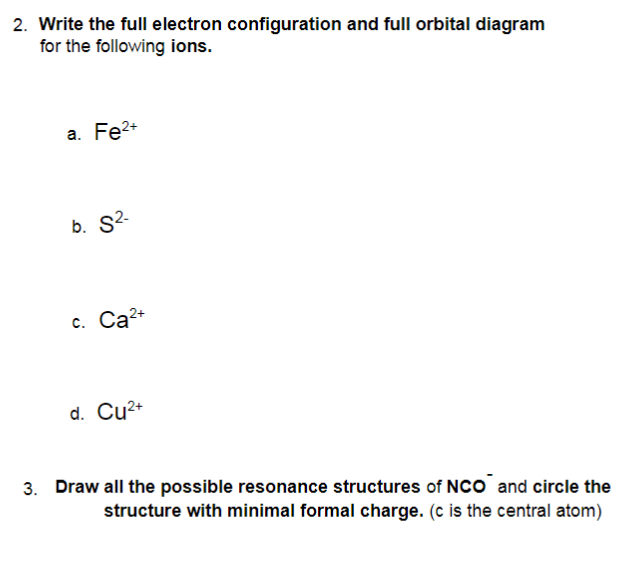
So now we'll work on problem 68 from chapter eight in this problem, just to draw orbital diagrams for four ions and indicate whether the ion is dia magnetic ...5 answers · 3 votes: Write orbital diagrams for each ion and determine if the ion is diamagnetic or paramagnetic.$$\text ...
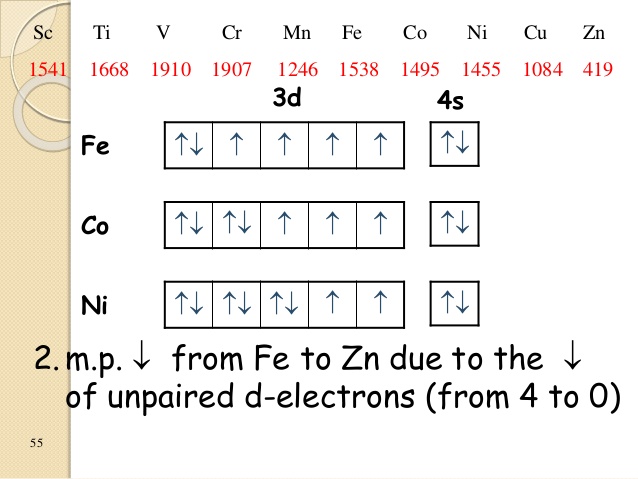
Fe2+ orbital diagram
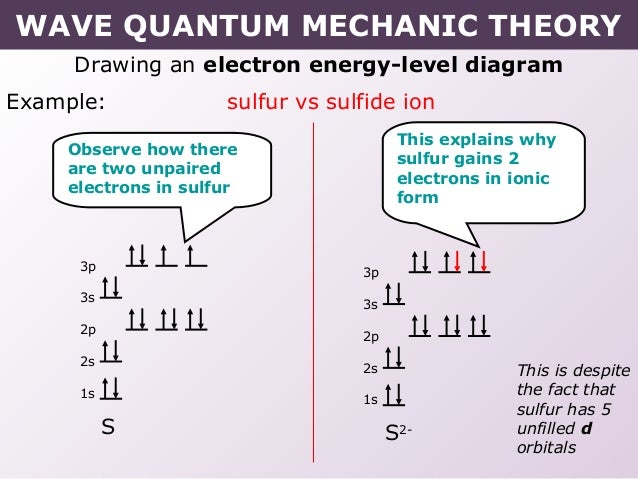
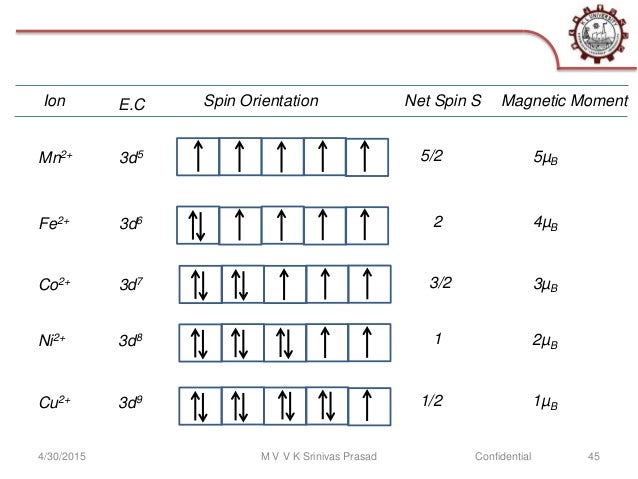
30 Mar 2018 · 9 answersThe configuration of neutral Fe atom is: 1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁶ 4s² where 4s electrons are the most energetic ones, therefore they are the first ones to be ...What is the electron configuration of Fe3+ and why ...10 answers29 Oct 2017Is the electronic configuration of Fe2+: [Ar] 3d6 or is ...1 answer15 Jul 2015What is the number of unpaired electrons in an Fe2+ ion?6 answers7 Mar 2018Which is more stable, Fe2+ or Fe3+? - Quora14 answers7 May 2017More results from www.quora.com 1 answerAs we can see from the above orbital diagram, Fe2+ 2 + has 4 unpaired electrons and Fe3+ 3 + has 5 unpaired electrons. In... In writing the electron configuration for Iron the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons ...5 Jul 2019 · Uploaded by Wayne Breslyn
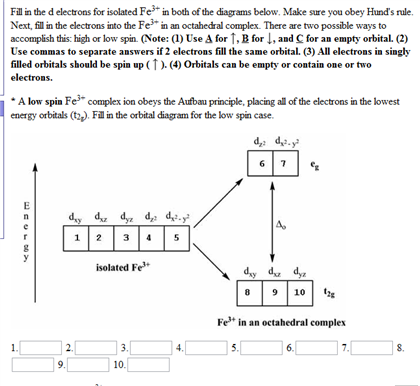
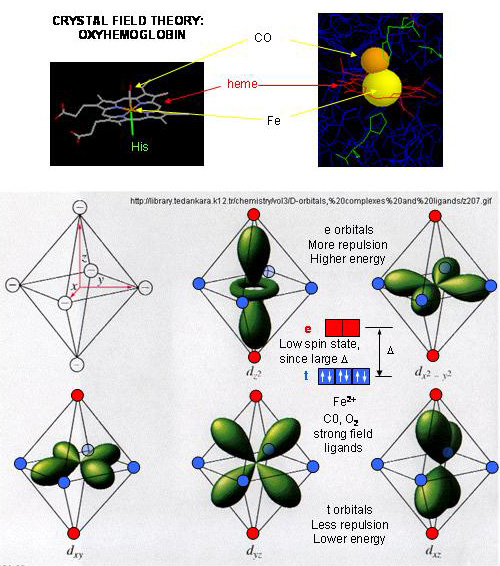
Fe2+ orbital diagram. It is not clear whether Fe2+ and Mn3+ ... Mn (e.g., Fe2+ - Mn3*, Mn3* - Fe3*, Mn2+ - Fe3*) seem ... Molecular orbital diagram for the Fe3*Mn2*O,o clus-.6 pages Select the correct valence orbital diagram for the Fe2+ ion and for the Fe3+ ion. Step-by-step solution. Step 1 of 3. The representation of the distribution ... In writing the electron configuration for Iron the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons ...5 Jul 2019 · Uploaded by Wayne Breslyn 1 answerAs we can see from the above orbital diagram, Fe2+ 2 + has 4 unpaired electrons and Fe3+ 3 + has 5 unpaired electrons. In...
30 Mar 2018 · 9 answersThe configuration of neutral Fe atom is: 1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁶ 4s² where 4s electrons are the most energetic ones, therefore they are the first ones to be ...What is the electron configuration of Fe3+ and why ...10 answers29 Oct 2017Is the electronic configuration of Fe2+: [Ar] 3d6 or is ...1 answer15 Jul 2015What is the number of unpaired electrons in an Fe2+ ion?6 answers7 Mar 2018Which is more stable, Fe2+ or Fe3+? - Quora14 answers7 May 2017More results from www.quora.com
I am in college General Chemistry, and we did [this lab](https://www.dropbox.com/s/1c0n58jrrps5uzp/Lab%207%20Magnetic%20Nanoparticles.pdf?dl=0) despite not covering the material in lecture, so I really have no idea how to go about it. Here are the questions I was given: Write the equation and net ionic equation for the observed reactions in Part 1. 2. Define the limiting agent in the reaction. And in what excess of ammonia was the magnetite (Fe3O4) synthesized, i.e. 2-fold, 5-fold, 10-fold...
I am in college General Chemistry, and we did [this lab](https://www.dropbox.com/s/1c0n58jrrps5uzp/Lab%207%20Magnetic%20Nanoparticles.pdf?dl=0) despite not covering the material in lecture, so I really have no idea how to go about it. Here are the questions I was given: Write the equation and net ionic equation for the observed reactions in Part 1. 2. Define the limiting agent in the reaction. And in what excess of ammonia was the magnetite (Fe3O4) synthesized, i.e. 2-fold, 5-fold, 10-fold...

































0 Response to "39 fe2+ orbital diagram"
Post a Comment