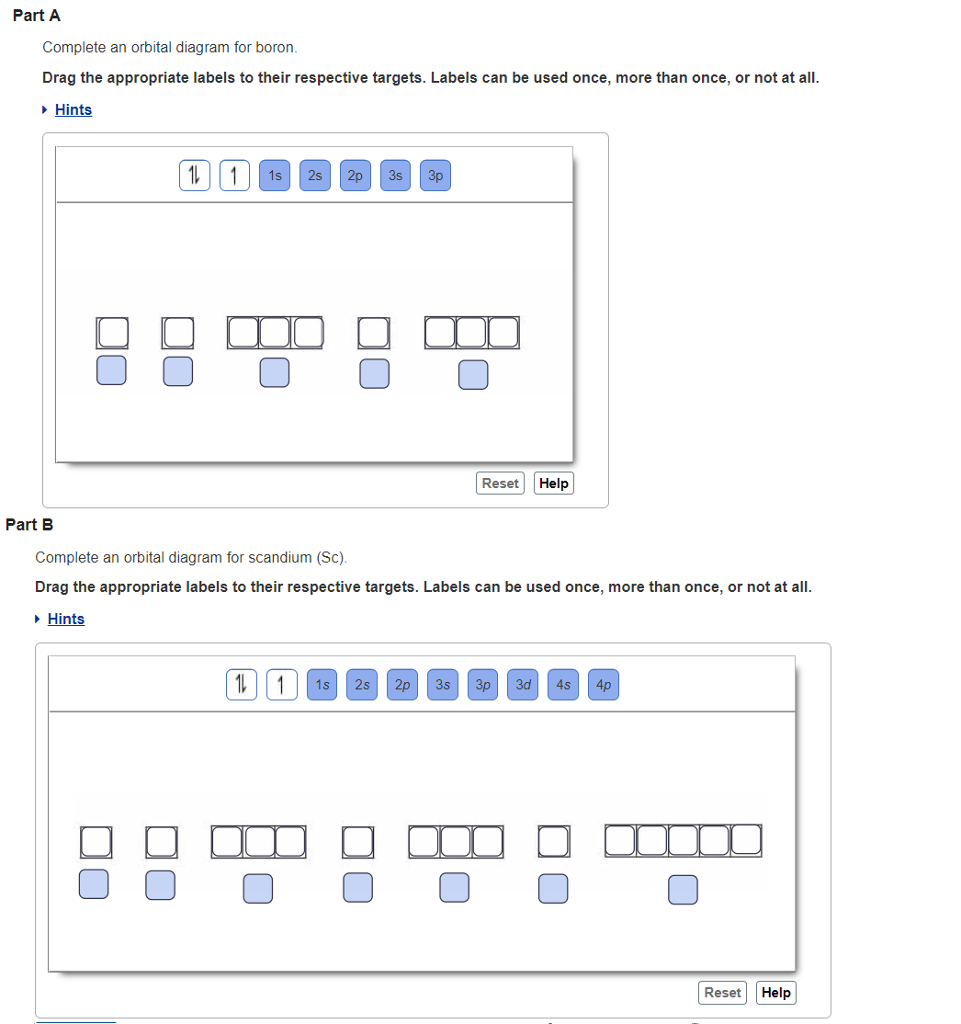
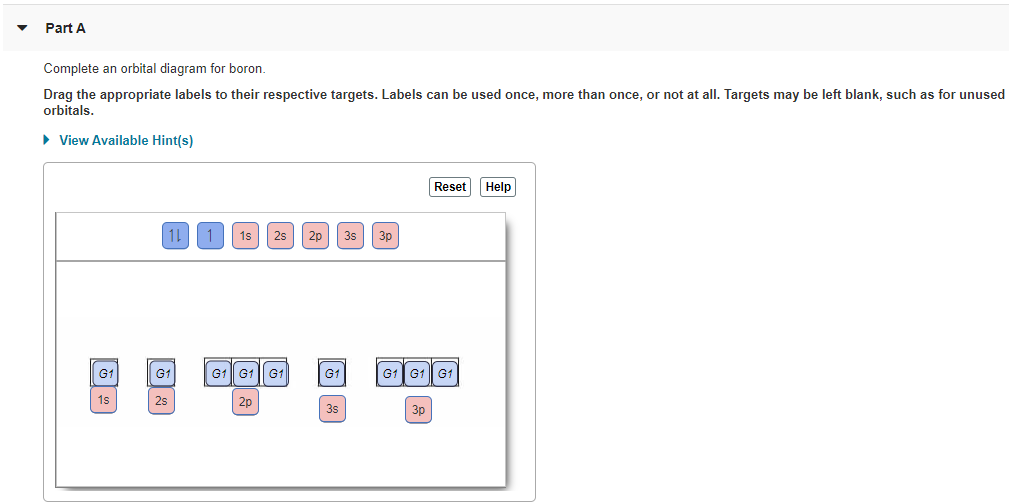
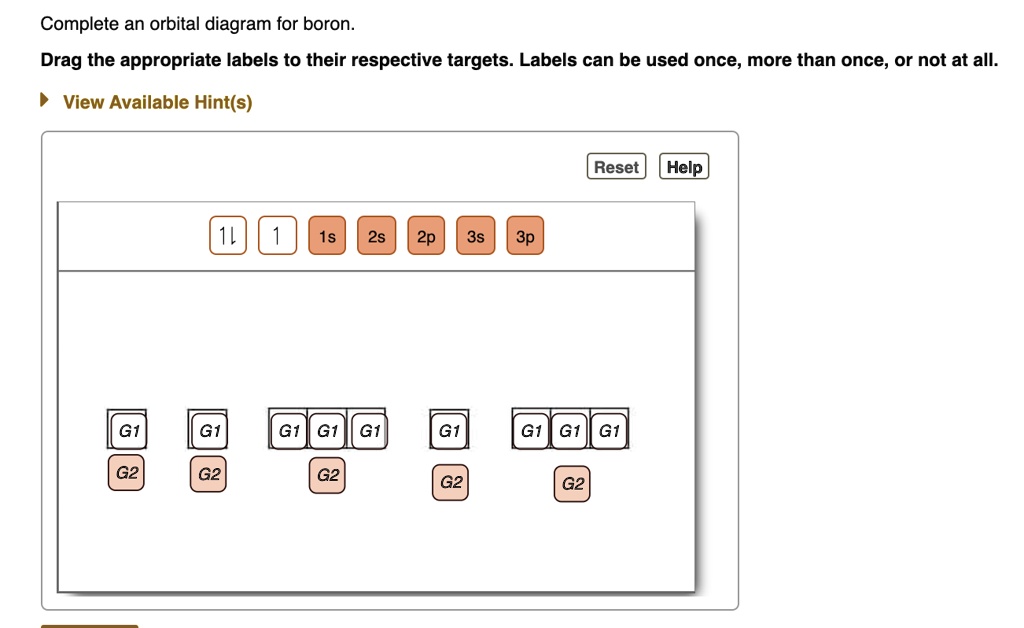
39 complete an orbital diagram for boron
The Aufbau principle is that the electrons present in the atom will first complete the lowest energy orbital and then gradually continue to complete the higher energy orbital. These orbitals are named s, p, d, f. The Aufbau electron configuration method is 1 s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d. When 3d orbital is complete, the new electron will enter the (a) 4p-orbital (b) 4f-orbital (d) 4s-orbital (d) 4d-orbital. Ans. According to the Aufbau Principle definition, the electron will enter from a lower energy shell to a higher energy level. 4p orbital is the next higher energy level than 3d orbital, so the electron will enter into 4p ...
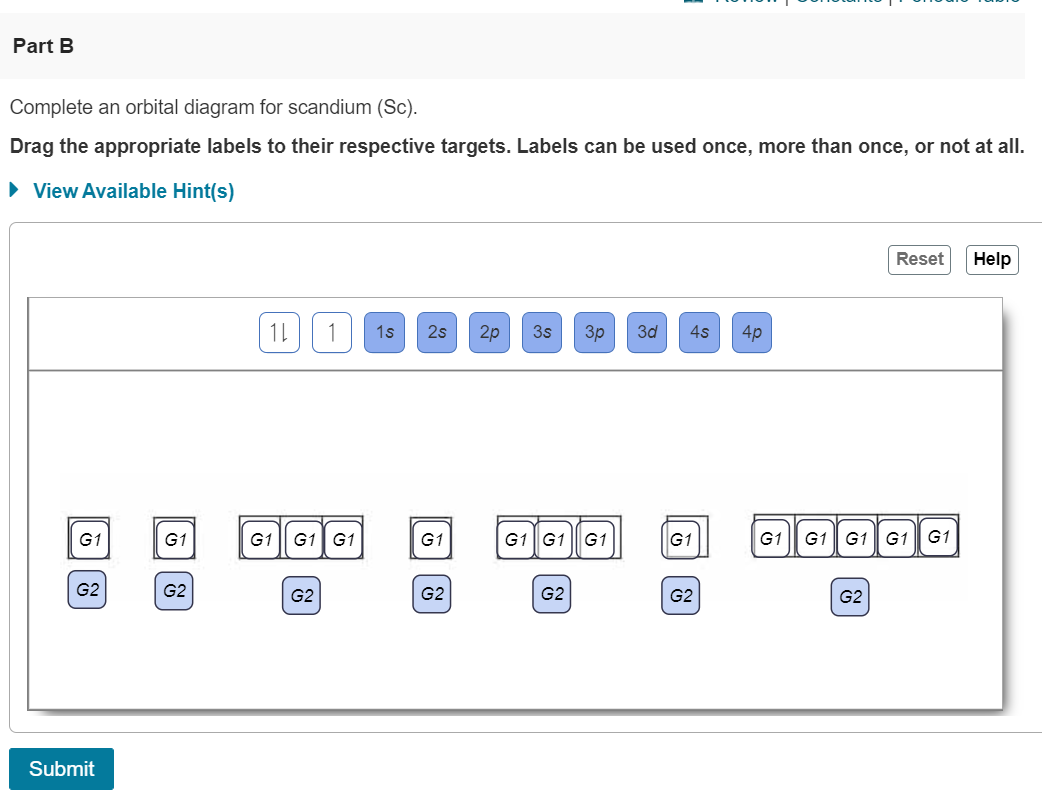
Solved Complete an orbital diagram for boron. Drag the | Chegg.com. Complete an orbital diagram for boron. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Complete an orbital diagram for scandium (Sc). Drag the appropriate labels to their respective targets.
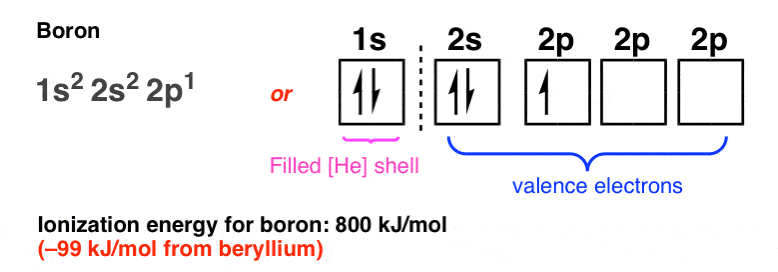
Complete an orbital diagram for boron
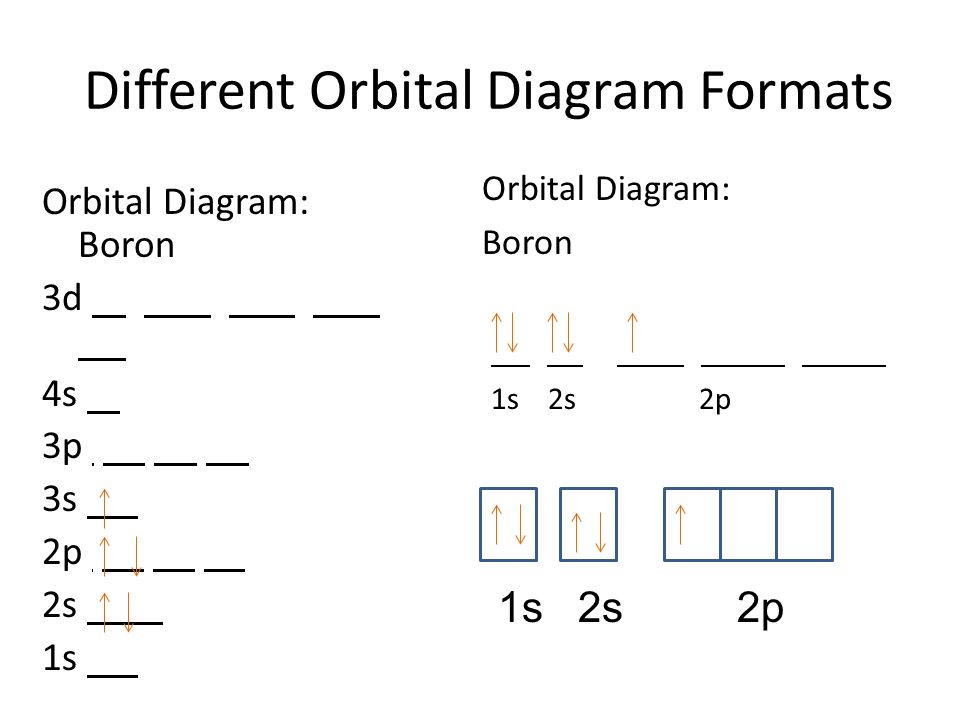
The complete electron configuration of arsenic, element 33, is _____. 4d. ... which explains why boron is larger than oxygen. Estimate the approximate Zeff felt by a valence electron of boron and oxygen, respectively? ... draw a ground state orbital diagram for Ar. orbital diagram (orbital box diagram) : 1s box has 2 arrows (as per helium above), 2s box has 2 arrows as per boron above, but now we see that there are 3 orbitals that make up the p-subshell (p x, p y, p z), into which we need to place 3 arrows. So, we apply Hund's Rule so that we maximise the number of unpaired electrons in all the 2p ... Complete an orbital diagram for boron. Boron is the fifth element with a total of 5 electrons. Use this tool to draw the orbital diagram. Therefore the b electron configuration will be 1s22s22p1. Lower energy subshells fill before higher energy subshells. Use the buttons at the top of the tool to add orbitals.
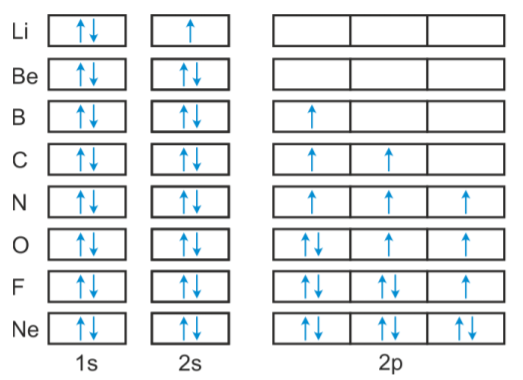
Complete an orbital diagram for boron. The orbital diagram in Model 3 is higher in energ than the ground state because ther is an electron in the 3 orbital that should be in a 2P orbital. The electron would need to have higher potential ennu to be in the 3s orbital. Read This! An state electron configuration … Nov 01, 2021 · Orbital diagram of Hydrogen (H) 2: Orbital diagram of Helium (He) 3: Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11 ... Isotopes of Boron (click to see decay chain): 6 B 7 B 8 B 9 B 10 B 11 B 12 B 13 B 14 B 15 B 16 B 17 B 18 B 19 B 9 B A molecular orbital energy level diagram just shows the energy levels in the molecule. Frequently, but not always, energy level diagrams are shown without any pictures of the orbitals, in order to focus attention on the energy levels, which in a fundamental way are the most important part of the picture.
Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Use the buttons at the top of the tool to add %(8). Question: Orbital Diagrams Draw an orbital diagram for boron. Use this tool to draw the orbital diagram. Boron is the fifth element with a total of 5 electrons. In writing the electron configuration for Boron the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for B goes in the 2s orbital. The remaining electron will go in the 2p orbital. Therefore the B electron configuration will be 1s 2 ... Neon electron configuration is 1s 2 2s 2 2p 6.The symbol for neon is ‘Ne’ and it is an inert element. This article gives an idea about the electron configuration of neon(Ne) and orbital diagram, period and groups, valency and valence electrons of neon, application of different principles. The tenth element in the periodic table is neon. Nov 17, 2021 · Molecular orbital diagram practice worksheet
Boron electron configuration is 1s 2 2s 2 2p 1.The period of boron is 2 and it is a p-block element. This article gives an idea about the electron configuration of boron(B) and orbital diagram, period and groups, valency and valence electrons of boron, application of different principles.. The fifth element in the periodic table is the boron(B). Problem: Part A. Complete an orbital diagram for boron.Draw orbital diagrams, and use them to derive electron configurationsTo understand how to draw orbital diagrams, and how they are used to write electron configurations.The electron configuration of an element is the arrangment of its electrons in their atomic orbitals. Electron configurations can be used to predict most of the chemical ... Complete an orbital diagram for boron. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. 1s: 2 arrows 2s: 2 arrows 2p: 1 arrow in the first orbital (leave the rest empty) Complete an orbital diagram for scandium (Sc). This problem has been solved! See the answer. See the answer See the answer done loading. Complete an orbital diagram for boron. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Complete an orbital diagram for scandium (Sc). Drag the appropriate labels to their respective targets.
Sep 22, 2021 · Electron configurations and orbital diagrams are used to show the arrangement of electrons in shells (levels), subshells (sublevels) and orbitals for specific atoms. Write complete electron configurations and abbreviated orbital diagrams for each of the elements given below.
In this video, we will explore the rules to follow when drawing orbital diagrams. We also determine the orbital diagram of boron.
Complete an orbital diagram for boron. Boron is the fifth element with a total of 5 electrons. Use this tool to draw the orbital diagram. Therefore the b electron configuration will be 1s22s22p1. Lower energy subshells fill before higher energy subshells. Use the buttons at the top of the tool to add orbitals.
Solved Draw A Complete Molecular Orbital Diagram Of Boron And Nitrogen Include Atomic Orbitals And Calculate Bond Order Of Bn And Bn Which Is Paramagnetic Which Has A Higher Bond Energy And
orbital diagram (orbital box diagram) : 1s box has 2 arrows (as per helium above), 2s box has 2 arrows as per boron above, but now we see that there are 3 orbitals that make up the p-subshell (p x, p y, p z), into which we need to place 3 arrows. So, we apply Hund's Rule so that we maximise the number of unpaired electrons in all the 2p ...
The complete electron configuration of arsenic, element 33, is _____. 4d. ... which explains why boron is larger than oxygen. Estimate the approximate Zeff felt by a valence electron of boron and oxygen, respectively? ... draw a ground state orbital diagram for Ar.
Write Electronic Configuration Of Following Elements In Form Of Orbital Notation And Orbital Diagram C Chemistry Topperlearning Com N0khl8gg
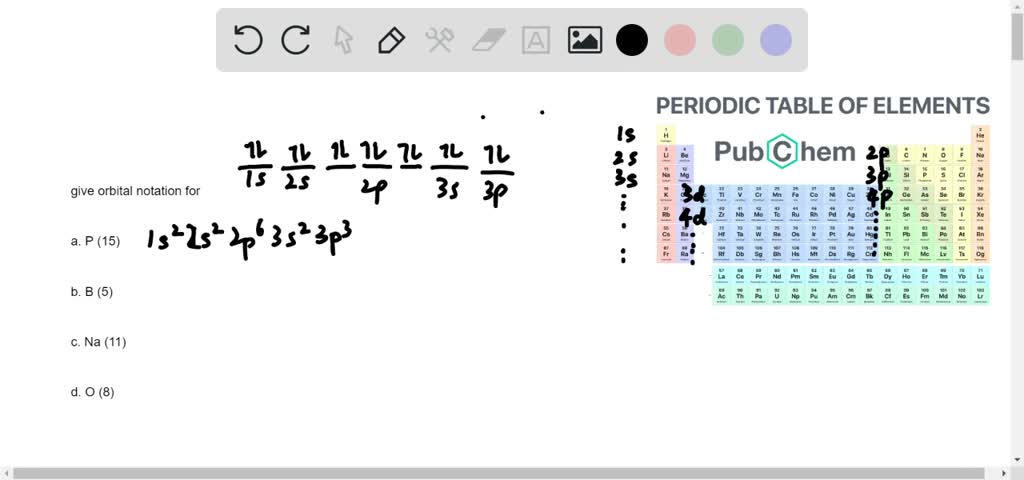
Solved Write The Orbital Notation For The Following Elements Hint See Sample Problem A A P B B C Na D O

Part A Write Orbital Diagrams For The Valence Electrons Of Ne Drag The Appropriate Labels To Homeworklib

Complete Orbital Diagrams Boxes With Arrows In Them To Represent The Electron Configuration Of Valence Homeworklib

Complete An Orbital Diagram For Boron Drag The Appropriate Labels To Their Respective Targets Labels Can Homeworklib








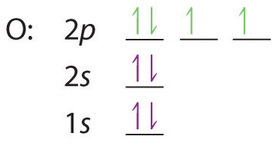











0 Response to "39 complete an orbital diagram for boron"
Post a Comment