38 lewis dot diagram h2o
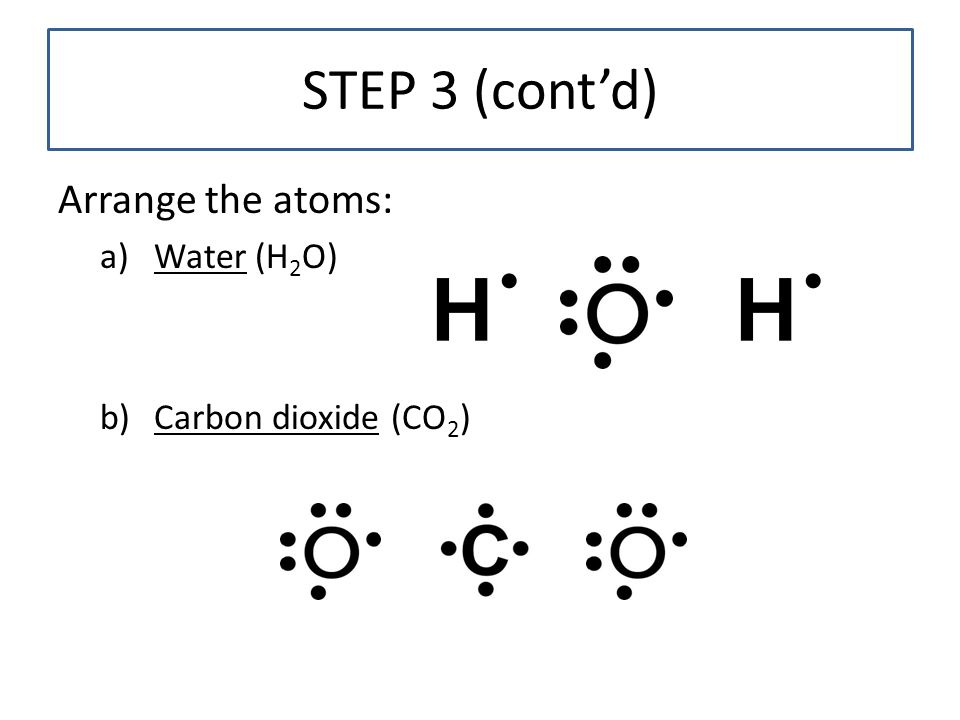
Lewis Dot of Water H2O - Kentchemistry.com 70 More Lewis Dot Structures. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule. The exception, of course, being the hydrogen's. They follow the duet rule (2 electrons). Water is a transparent, tasteless, odorless liquid at room temperature and standard pressure. Water is a polar molecules. Solved Draw a Lewis dot diagram for water (H2O) b) Draw a ... This problem has been solved! Draw a Lewis dot diagram for water (H2O) b) Draw a Lewis dot diagram for methane (CH4) c) Draw a Lewis dot diagram for ammonia (NH3) Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high.
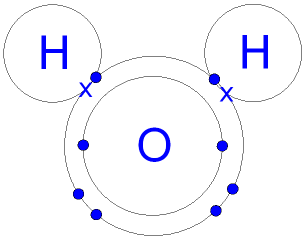
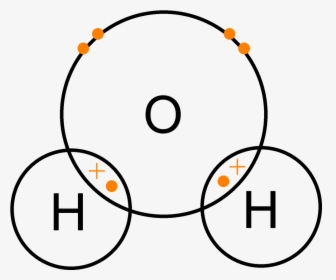
Lewis Dot Diagram H2o - schematron.org Apr 04, 2019 · This is the Lewis Dot Structure for H2O. You could alternatively also draw the structure by including two dots for every bond. While oxygen's octet seems to have.The Lewis dot structure of water begins with a single O atom in the center. On the right and left sides are a singly bonded H atom.
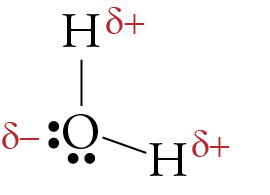
Lewis dot diagram h2o
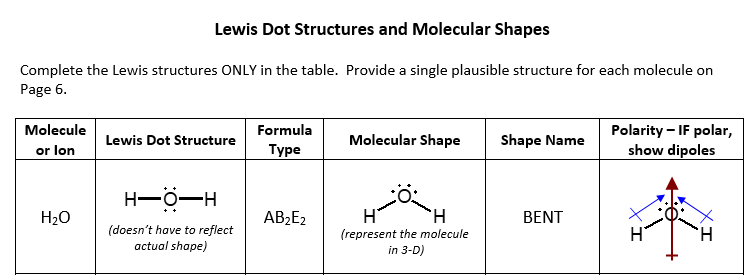
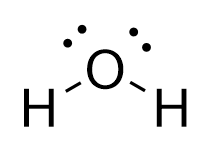
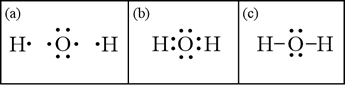
Lewis Structure of H2O (Water) - Drawing Steps In the lewis structure of H 2 O, there are two single bonds around oxygen atom. Hydrogen atoms are joint to oxygen atom through single bonds. Also, there are two lone pairs on oxygen atom. Water molecule is a simple molecule. Drawing lewis structure of water molecule is simple than some of other complex molecules or ions. MakeTheBrainHappy: The Lewis Dot Structure for H2O H2O's Lewis Dot Structure gives it many unique properties mostly due to the two lone pairs on the central oxygen atom. This increases electron-electron repulsion and therefore creates a bent structure as opposed to CO2's linear structure. This "bent" molecular structure gives it many unique properties such as being polar. What is the Lewis dot structure of H2O? - Answers See answer (1) Best Answer. Copy. See the image of the Lewis dot structure of water in the "sources and related links" section below. Wiki User. ∙ 2012-02-27 06:19:46. This answer is: Helpful ...
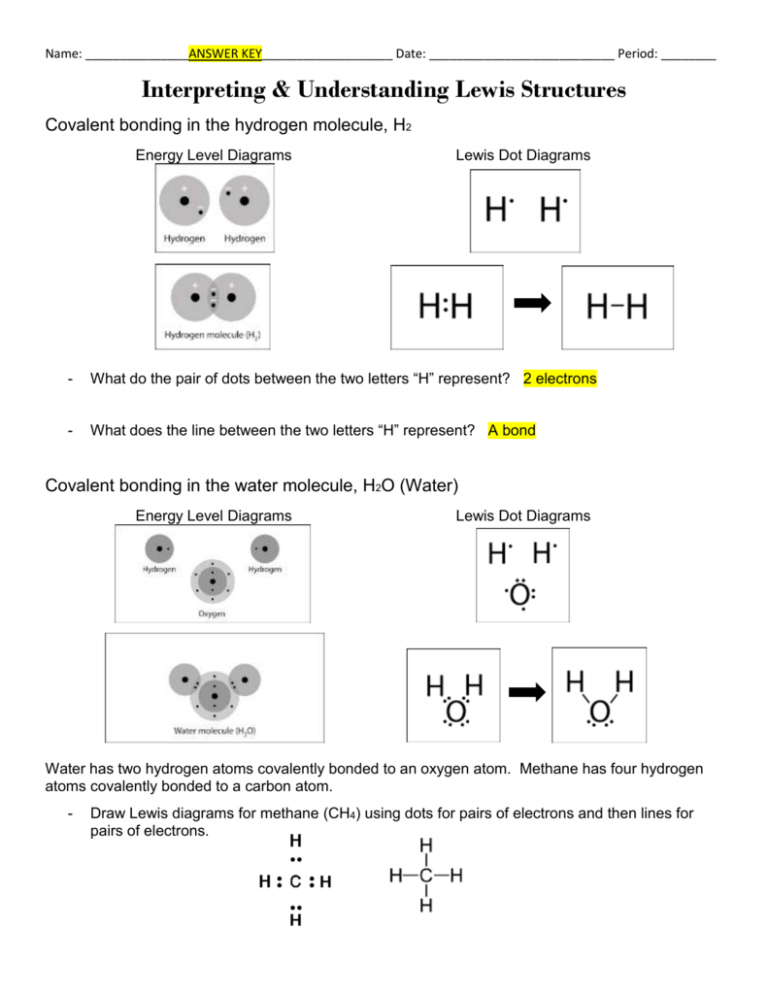
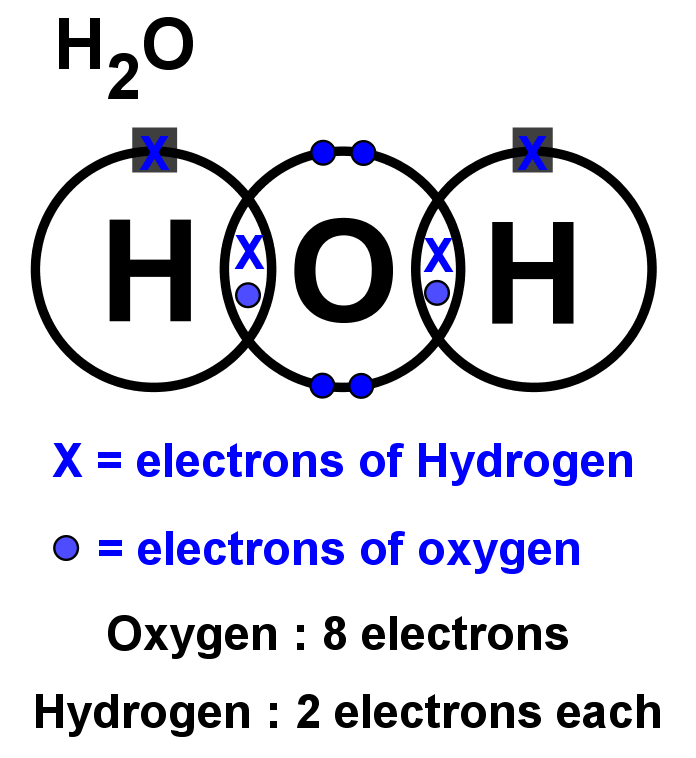
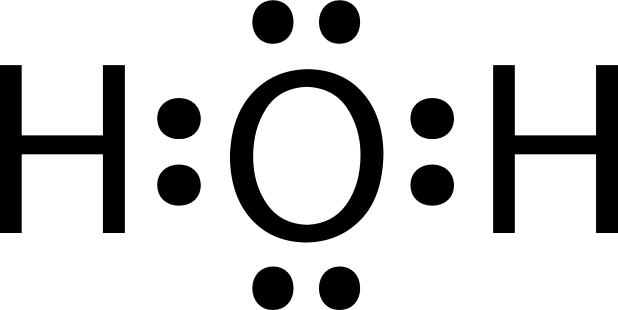
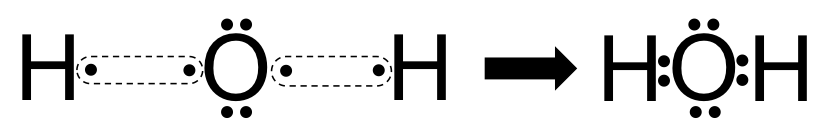
Lewis dot diagram h2o. How can I draw the Lewis structure for H2O? | Socratic Jul 15, 2014. You can find a procedure for drawing Lewis structures at this location. For H₂O, O must be the central atom. The skeleton structure is H-O-H. O has 6 valence electrons, and each H has one. You must arrange 8 electrons in pairs so that O has 8 and each H has two electrons in its valence shell. The trial structure is. What is the Lewis dot structure for h2o? - FindAnyAnswer.com Drawing the Lewis Structure for H2O You have a total of 8 valence electrons available to fill the octets of Oxygen and Hydrogen. Remember that Hydrogen only needs two electrons to have a full outer shell. It is helpful if you: Try to draw the H2O Lewis structure before watching the video. What is the structural formula of water? H2O How to find the Lewis dot diagram for H2O - Quora How can I draw the Lewis structure of 2 H2O on paper (I need it for the exam)? First off, write valence electrons for both elements Two of H have 2 valence electrons and O has 6. The sum is 8e. Lets draw a single bonds to attach 2H to O. H —— O —— H As we know, a bond requires 2 electrons to maintain. So 4e were used. 6 - 4 = 2 e. What is the Lewis structure of H2O? - Quora Originally Answered: What is a Lewis structure of H2O? A Lewis structure is a diagram that shows the bonding between the atoms of a molecule and any possible lone pairs of electrons. To ensure stability, most atoms require an octet - that is, 8 electrons in their outermost electron shell. Hydrogen, however, is an exception.
Covalent bond and Lewis dot structure (H2O & CO2) (video ... Covalent bond and Lewis dot structure (H2O & CO2) Google Classroom Facebook Twitter. Email. Bonding in carbon- covalent bond. Carbon and hydrocarbons. Covalent bond . Covalent bond and Lewis dot structure (H2O & CO2) This is the currently selected item. Single and multiple covalent bonds. Lewis Dot Structure of H2O, (Water) - YouTube I quickly take you through how to draw the Lewis Structure of water, H2O. I also go over hybridization, shape and bond angle. H2O2 lewis structure, molecular geometry, hybridization ... The H2O2 lewis dot structure is very simple and the procedure for drawing it same as the other molecules.. Let's see how to draw its Lewis structure step by step.. Follow some steps to drawing the H2O2 Lewis dot structure 1. Count total valence electron in H2O2. In the first step, we need to calculate how many valence electrons are present in it. Lewis Dot Structure for H2O | Chemical Bonding | Success ... Watch the video of Dr. B. drawing the Lewis dot structure for H2O and answer the questions below. The H 2 O Lewis dot structure is seen fairly frequently. A common error it to put two oxygen atoms and one hydrogen making HO 2. Make sure you have two hydrogens and one oxygen in H 2 O! Hint: look at the questions before watching the video.
Water Lewis Structure - How to Draw the Lewis Structure ... ----- Steps to Write Lewis Structure for compounds like H2O ----- 1. Find the total valence electrons for the H2O molecule. 2. Put the least electronegative atom in the center. Note: Hydrogen (H)... Lewis acids and bases - Wikipedia Depicting adducts. In many cases, the interaction between the Lewis base and Lewis acid in a complex is indicated by an arrow indicating the Lewis base donating electrons toward the Lewis acid using the notation of a dative bond — for example, Me 3 B ← NH 3.Some sources indicate the Lewis base with a pair of dots (the explicit electrons being donated), which allows … CH2O lewis structure, molecular geometry, bond angle ... Formaldehyde (CH2O) lewis dot structure, molecular geometry, polar or non-polar, hybridization. Formaldehyde is an organic compound that appears as a colorless gas with the chemical formula CH2O. It is the simplest aldehyde made up of two hydrogens, one carbon, and one oxygen. It is widely used as a preservative because of its antibacterial ... Lewis Dot Diagram For H2o2 - schematron.org When drawing diagrams, hydrogen always goes on the outside. So you would put down an O with an H on each side. Now each hydrogen only needs 2 valence electrons, so you would draw 2 dots on each side of the O, between it and the H. So you have 4 left over, so put two dots on the top, and two on the bottom. So that is the Lewis dot structure.
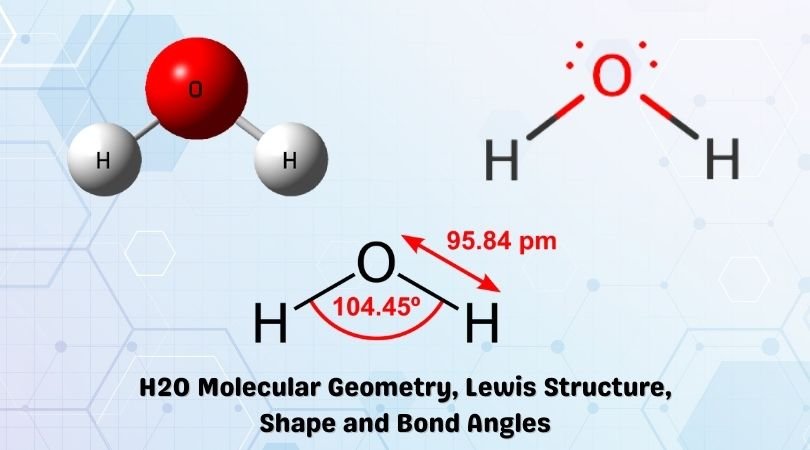
H2O Molecular Geometry, Lewis Structure, Shape and Bond Angles This is the Lewis structure of the H2O molecule that has two single bonds between Oxygen and Hydrogen. As a result, there are two lone pairs in this molecule and two bonding pairs of electrons. H2O Hybridization When two atoms share electrons and form bonds, there is the formation of hybridized orbitals.
C2H5OH Lewis Structure - Lewis Dot Structure | Chem Helps CH3CH2OH Lewis Dot Structure is a complex structure. It's not easy to draw if you are not familiar with the topic. You should be checking H2O Lewis Structure or CO Lewis Structure first to get familiar with Lewis Structures before you try to draw CH2CH2OH Lewis Dot Structure .
Draw Lewis dot diagram for the following. Water (H2O ... Draw Lewis dot diagram for the following. Water (H2O) Maharashtra State Board HSC Science (Computer Science) 11th. Textbook Solutions 6916. Important Solutions 17. Question Bank Solutions 4559. Concept Notes & Videos 336. Syllabus. Advertisement Remove all ads. Draw Lewis dot diagram for the following. ...
(PDF) Essentials of Physical Chemistry by B.S. Bahl Arun ... Preface The Essentials of Physical Chemistry has been written for BSc students. It has been national bestseller for more than 65 years. It has been used by more than 2 million students. It is 26 editions old. It really has been that long. A lot of
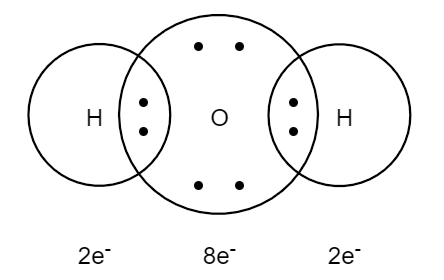
Water Lewis Structure: How to Draw the Dot Structure for ... Let's do the Lewis structure for water: H2O. On the periodic table, Hydrogen's in group 1, it has 1 valence electron; but we have two of them, so let's multiply that by 2. And Oxygen is in group 6, sometimes called 16, so it has 6 valence electrons. So 1 times 2 is 2, plus 6; 2 plus 6 equals 8. We have a total of eight valence electrons.
Free PDF Download - Handbook-of-Chemistry-and-Physics ... Enter the email address you signed up with and we'll email you a reset link.
H2O Lewis Structure, Molecular Geometry, and Hybridization 2 days ago · Draw the lewis diagram: The Geometrical Structure of the H2O molecule The bond angle among hydrogen-oxygen-hydrogen atoms (H-O-H) is 104.5°. From this, it can be understood that the geometrical structure of a single H2O molecule is bent.
Lewis dot diagrams for the following:(a) Hydrogen (H2) (b ... Click here👆to get an answer to your question ️ Lewis dot diagrams for the following:(a) Hydrogen (H2) (b) Water (H2O) (c) Carbon dioxide (CO2) (d) Methane (CH4)
Lewis Structures - Lewis Dot Structure | Chem Helps Lewis Structure is the representation of shared electrons with lines between atoms. A schematic representation of the molecule is made. In the Lewis Dot Structure, if one electron is shared between two atoms, a single line is drawn, if two electrons are used together, a double line, and if three electrons are used together, three lines are drawn.
Lewis Dot Diagram H2o Dec 24, 2018 · The Lewis dot structure for water shows the electron from hydrogen and an electron from oxygen being shared in a covalent bond. The other four valence electrons in oxygen are in pairs at the bottom. The Lewis dot structure of water begins with a single O atom in the center. On the right and left sides are a singly bonded H atom.
Lewis Electron Dot Structures - Detailed Explanation with ... Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. They also display the total number of lone pairs present in each of the atoms that constitute the molecule. Lewis dot structures are commonly referred to as electron dot structures or Lewis structures.
What Is The Correct Lewis Structure For Water What is the correct Lewis structure for H2O? The skeleton structure is H-O-H. O has 6 valence electrons, and each H has one. You must arrange 8 electrons in pairs so that O has 8 and each H has two electrons in its valence shell. You have eight valence electrons in your trial structure, so it has the correct number of electrons.
What is the Lewis dot structure of H2O? - Answers See answer (1) Best Answer. Copy. See the image of the Lewis dot structure of water in the "sources and related links" section below. Wiki User. ∙ 2012-02-27 06:19:46. This answer is: Helpful ...
MakeTheBrainHappy: The Lewis Dot Structure for H2O H2O's Lewis Dot Structure gives it many unique properties mostly due to the two lone pairs on the central oxygen atom. This increases electron-electron repulsion and therefore creates a bent structure as opposed to CO2's linear structure. This "bent" molecular structure gives it many unique properties such as being polar.
Lewis Structure of H2O (Water) - Drawing Steps In the lewis structure of H 2 O, there are two single bonds around oxygen atom. Hydrogen atoms are joint to oxygen atom through single bonds. Also, there are two lone pairs on oxygen atom. Water molecule is a simple molecule. Drawing lewis structure of water molecule is simple than some of other complex molecules or ions.


/ScreenShot2018-11-19at11.40.52PM-5bf3909a46e0fb00510dbd6d.png)
















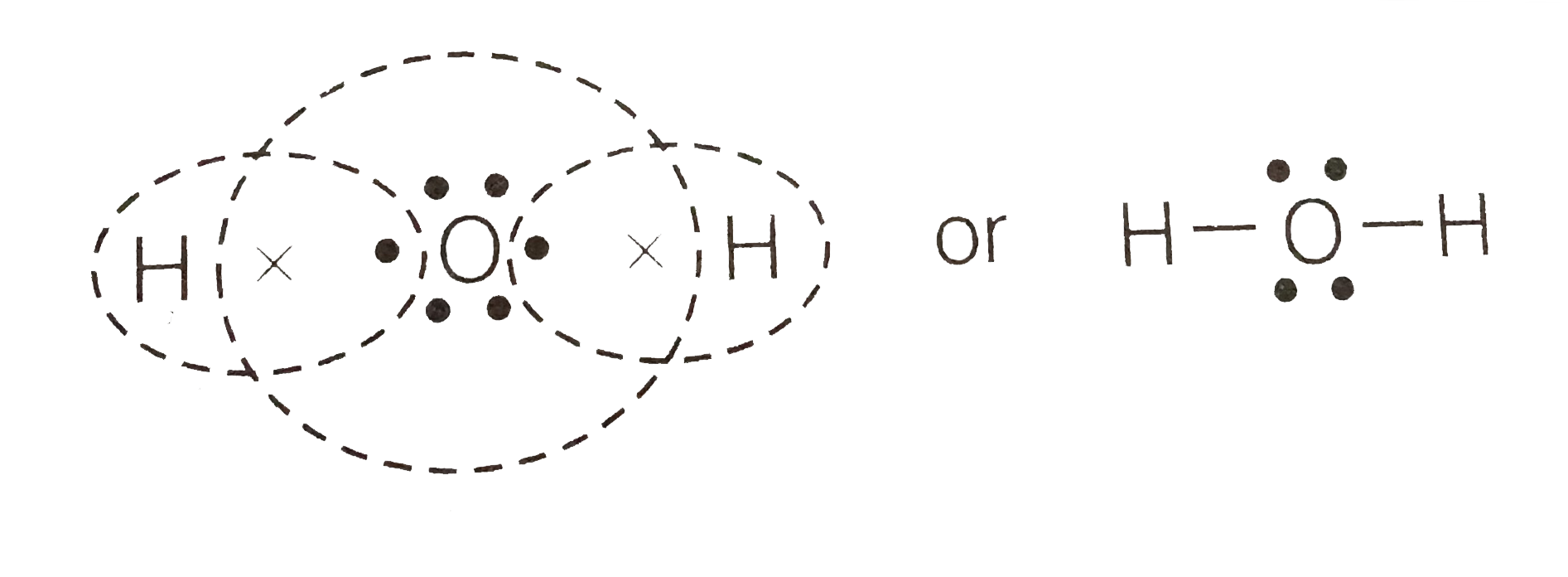

0 Response to "38 lewis dot diagram h2o"
Post a Comment