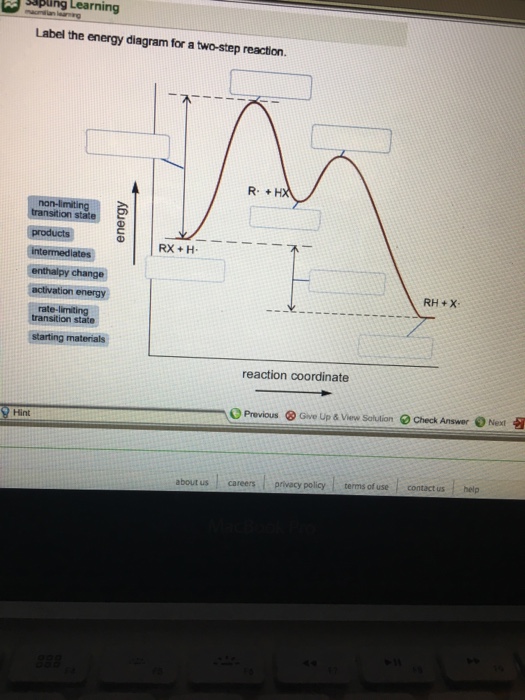
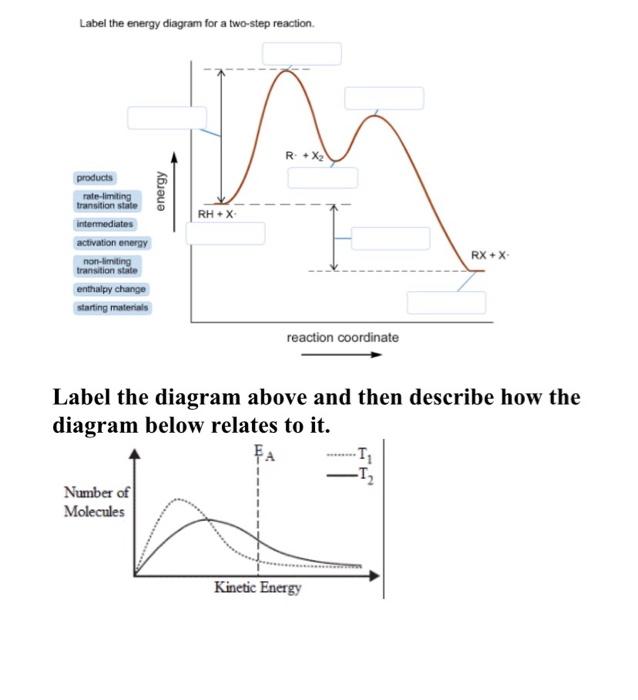
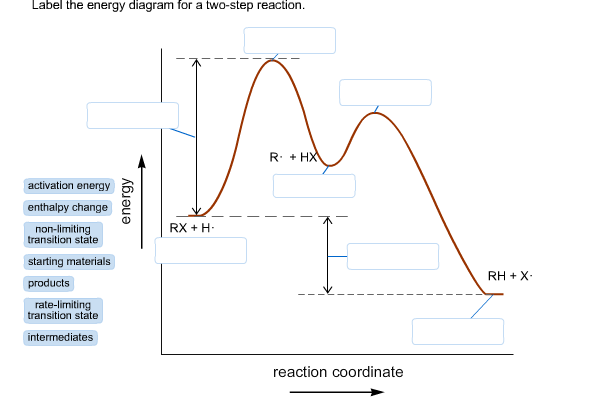
42 label the energy diagram for a two-step reaction.
Potential Energy Diagrams - Kentchemistry.com A potential energy diagram plots the change in potential energy that occurs during a chemical reaction. This first video takes you through all the basic parts of the PE diagram. YouTube. Sometimes a teacher finds it necessary to ask questions about PE diagrams that involve actual Potential Energy values. Visualize the reaction energy diagram for a one-step ... Draw a reaction-energy diagram for a two-step endothermic reaction with a rate-limiting second step. CHEMISTRY. Draw a reaction-energy diagram for a one-step exothermic reaction. Label the parts that represent the reactants, products, transition state, activation energy, and heat of reaction.
Energy Diagrams: Describing Chemical Reactions Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DGrxn, as well as DG1* and DG2* for the first and second activation energies, respectively. Label the positions corresponding to the transition states with an asterisk.
Label the energy diagram for a two-step reaction.
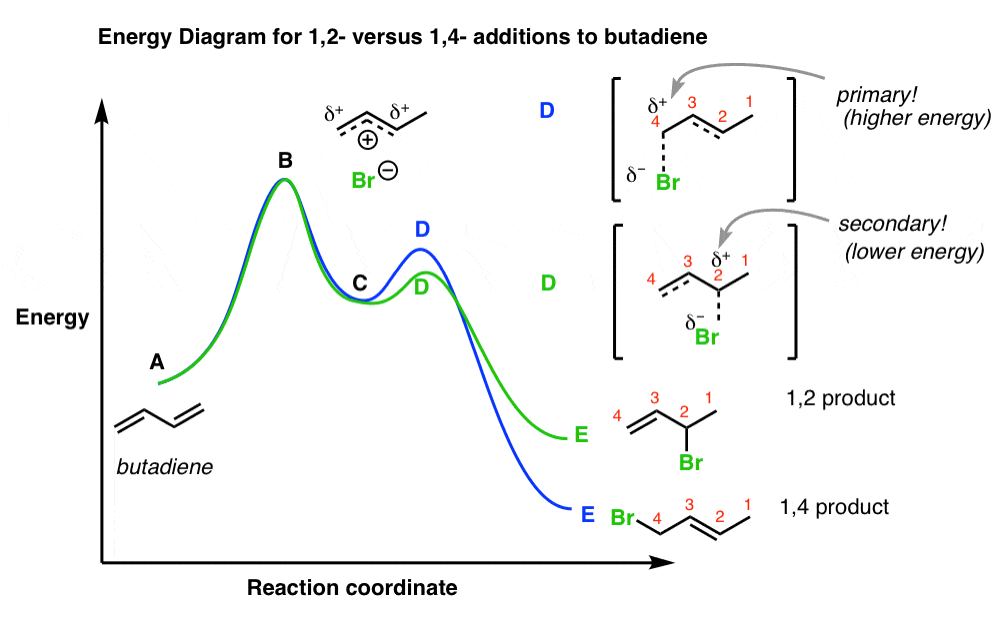
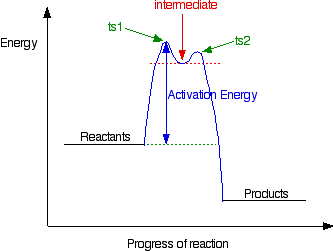
Energy Diagram for a Two-Step Reaction Mechanism by Ashima Singh Energy Diagram for a Two-Step Reaction Mechanism Complete Energy Diagram for Two-Step Reaction A Two-Step Reaction Mechanism The transition states are located at energy maxima. The reactive intermediate B+ is located at an energy minimum. Each step has its own delta H and 1. On The Energy Versus Reaction Progress Diagram Below ... 1. On The Energy Versus Reaction Progress Diagram Below, Label The Reactants And Products, Any Transition States, And Reaction Intermediates, The Change In Enthalpy For The Overall Reactions, And Any Activation Energies. Potential Energy Reaction Progress Is The Reaction Exothermic Or Endothermic?... SOLVED:Draw a reaction coordinate diagram for a two-step ... Draw a reaction coordinate diagram for a two-step reaction in which the first step is endergonic, the second step is exergonic, and the overall reaction is endergonic. Label the reactants, products, intermediates, and transition states. Answer View Answer Discussion You must be signed in to discuss. Watch More Solved Questions in Chapter 3
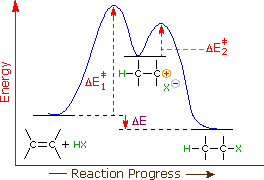
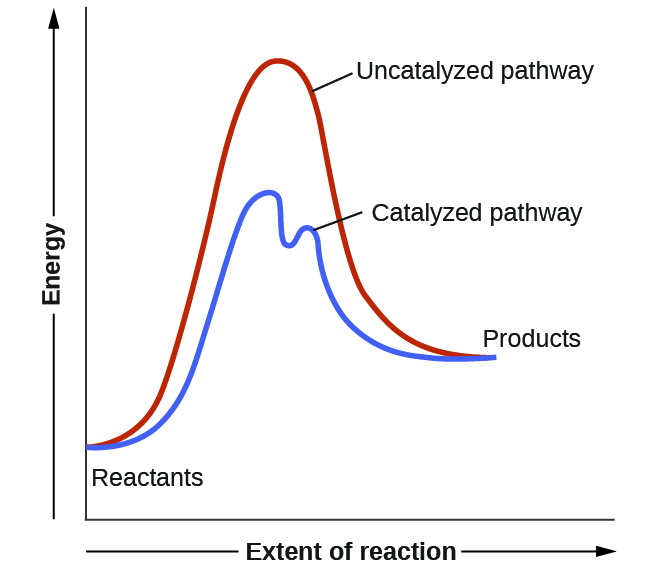
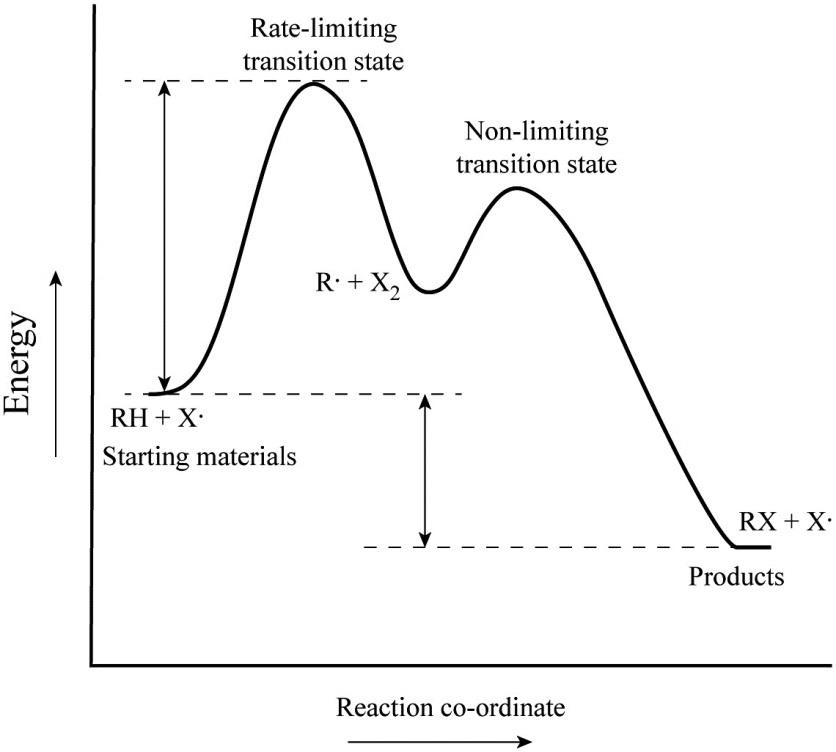
Label the energy diagram for a two-step reaction.. Solved Label the energy diagram for a two-step reaction. R. Question: Label the energy diagram for a two-step reaction. R. + HX Energy RX + H . RH + X Reaction coordinate Answer Bank enthalpy change intermediates ... PDF or CH CH ¥ + Br¥ CH CH Br Termination: Br¥ + Br¥ BrÐBr CH ... 1. Draw a reaction energy diagram for a two-step reaction in which the first step is endothermic, the second step is exothermic, the reaction is exothermic overall, and the second step is the rate-limiting step. Label the reactant, intermediate, product, and both transition states. Potential energy Reaction coordinate Reactant Intermediate ... Energy Diagrams of Two Step Reactions - YouTube Watch Complete videos @ Organic Chemistry 1 Energy Diagram Catalyzed Vs Uncatalyzed Reaction Below is an energy diagram illustrating the difference in a catalyzed reaction versus an uncatalyzed reaction. Label the energy diagram and answer the question that follows% (1). Catalyzed reactions have a lower activation energy (rate-limiting free energy of activation) than the corresponding uncatalyzed reaction, resulting in a higher ...
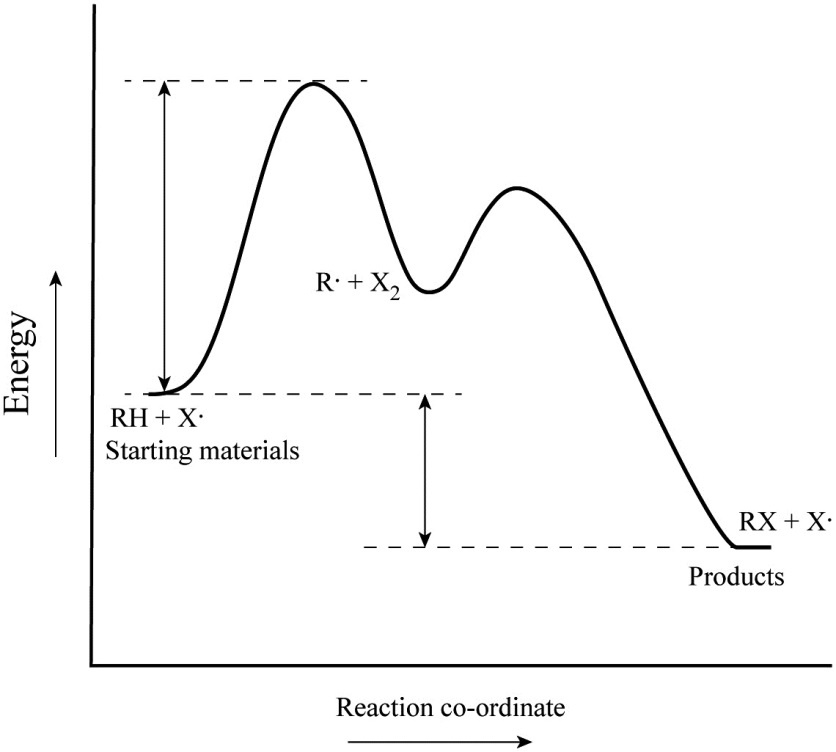
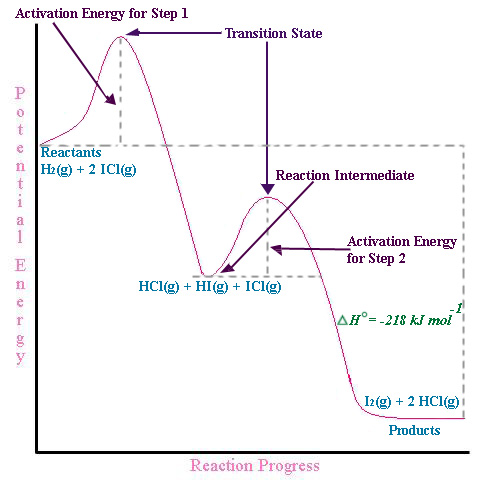
Label the energy diagram for a two-step reaction - Learn ... 19 Mar 2018 — An energy profile diagram is a theoretical representation that shows how the energy of the system changes during a chemical reaction. Some ... PDF Representing a Reaction with a Potential Energy Diagram Draw and label potential energy diagram for the reaction including a molecular structure that could represent an activated complex. The activated complex would show an unstable association of one CH 4(g) molecule and O 2(g) molecule with partial bonds. Check Your Solution The potential energy diagram should match the given information. Draw an energy diagram for a two-step reaction where the ... Draw an energy diagram for a two-step reaction where the first step is exothermic and the reaction overall is endothermic. The first step is rate limiting. Label reactants, products and intermediates. Multistep Reactions - Softschools.com The energy diagram of a two-step reaction is shown below. In the above reaction, a reactant goes through one elementary step with a lower activation energy (transition state 1) to form the intermediate. The intermediate then goes through a second step (transition state 2) with the highest energy barrier to form the product.
Label the energy diagram for a two-step reaction. - Chegg Answer to Solved Label the energy diagram for a two-step reaction. Question #62891 | Socratic The figure above represents the reaction profile of a two step, exothermic reaction. The y-axis represents the potential energy of the reaction species, and the x-axis represents the progress of the reaction. The reaction is exothermic because the energies of the products are lower than those of the reactants. The reactants are represented by the horizontal line at the far left of the graph ... Solved: Draw an energy diagram for a two-step reaction with ... Problem 21E Draw an energy diagram for a two-step reaction with Keq > 1. Label the overall Δ G °, transition states, and intermediate. Is Δ G ° positive or negative? Step-by-step solution 95% (19 ratings) for this solution Step 1 of 3 Gibb's free-energy is the energy difference between the reactants and the products. Journal of the American Chemical Society | Vol 144, No 11 14.3.2018 · Two nonplanar 2D covalent organic frameworks (COFs) with a square (c-HBC-COF) or kagome (DHP-COF) lattice are designed and synthesized from D2- and C4v-symmetric distorted aromatics with different π-conjugated structure. c-HBC-COF with more efficient π-stacking and large π-conjugation exhibits a photoconductivity and charge carrier mobility (up to …
Label the energy diagram for a two-step reaction. - Chegg Question: Label the energy diagram for a two-step reaction. This problem has been solved! See the answer ...
Label The Energy Diagram (7 Bins) And Indicate Which Reaction ... Aug 11, 2018 · A Two-Step Reaction Mechanism We draw an energy diagram for each step, and then combine them in an energy diagram for the overall two step mechanism. Label the energy diagram (7 bins) and indicate which reaction corresponds to the energy diagram. Label the energy diagram (7 bins) and indicate which reaction corresponds to the energy diagram.
Solved: Draw a reaction energy diagram for a two-step ... Problem 18P Draw a reaction energy diagram for a two-step reaction that has an endothermic first step and an exothermic second step. Label the reactants, transition states, reaction intermediate, activation energies, and enthalpy differences. Step-by-step solution 100% (5 ratings) for this solution Chapter 3, Problem 18P is solved. View this answer
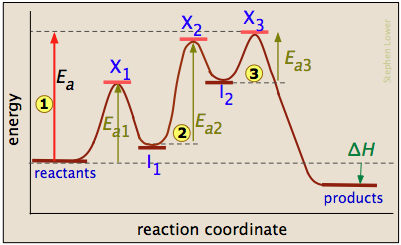
Welcome to CK-12 Foundation | CK-12 Foundation is the complex created in the first reaction, while is the activated complex created in the second reaction. Thus, for this two-step process, there are two activated complexes. Example: Draw the potential energy diagram for the following multi-step reaction . Properly label the diagram. Solution: Rate of Reaction is Determined by Slowest Step
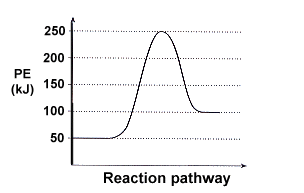
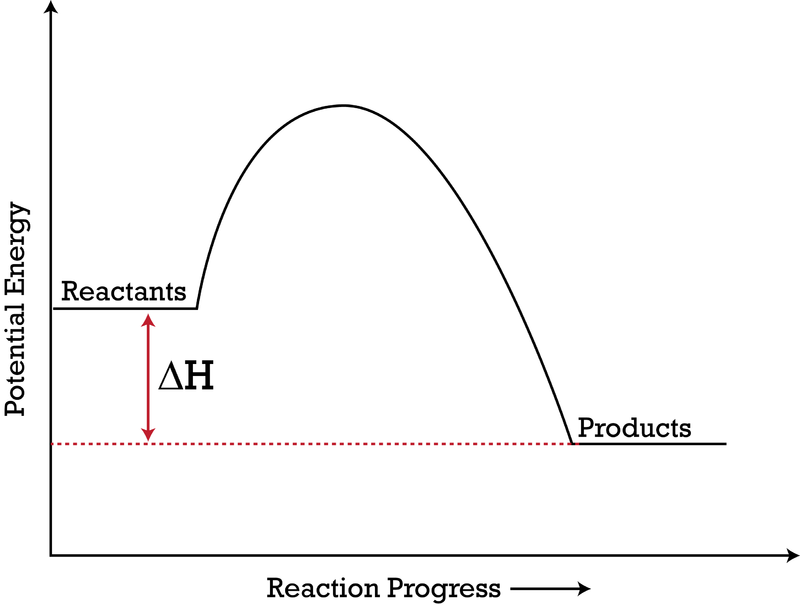
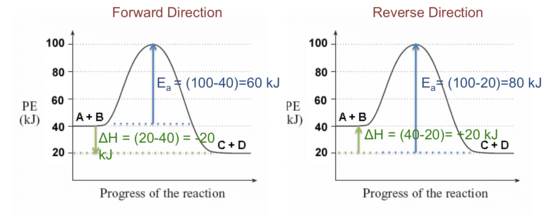
ENERGY PROFILES FOR SIMPLE REACTIONS - chemguide Diagrams like this are described as energy profiles. In the diagram above, you can clearly see that you need an input of energy to get the reaction going. Once the activation energy barrier has been passed, you can also see that you get even more energy released, and so the reaction is overall exothermic. If you had an endothermic reaction, a ...
(PDF) Engineering Chemistry by Jain & Jain - Academia.edu Academia.edu is a platform for academics to share research papers.
Label the energy diagram for a two-step reaction. - Chegg Question: Label the energy diagram for a two-step reaction. This problem has been solved! See the answer ...
PDF Potential Energy Diagram Notes - ms. adrangi's teaching site The P.E. diagram for these types of reaction is identified by having at least two 'bumps' Example 2 NO + H 2(g)→ N 2O + H 2O + heat Step #1 NO + NO → N 2O 2(fast) Step #2 N 2O 2(g)+ H 2(g)→ N 2O + H 2O (slow) AP Chemistry Name: Potential Energy DiagramsPotential Energy Diagrams
Solved Label the energy diagram for a two-step reaction ... Solved Label the energy diagram for a two-step reaction. | Chegg.com. Science. Chemistry. Chemistry questions and answers. Label the energy diagram for a two-step reaction. enthalpy change transition state starting materials RX+H products rate-limiting transition state intermediates activation energy reaction coordinate.
(PDF) General chemistry 5th edition | MOHD ... - Academia.edu (Northern Arizona University) and Raymond Chang, this success guide is written for use with General Chemistry. It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems.
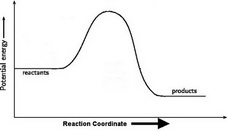
How to draw the potential energy diagram for this reaction ... C3H8(g) + 5O2(g) → 3CO2(g) +4H2O(g) + 2219.9 kJ, we say that ΔH ∘ C = − 2219.9 kJ/mol propane. We approximate that this is the change in potential energy for the reactants going to the products. The above is for an endothermic reaction.
Answered: Draw a reaction energy diagram for a… | bartleby Draw a reaction energy diagram for a two-step reaction with the first step is the slow step and the reaction is endothermic. Label the parts of the diagram corresponding to reactants, products, transition states, intermediates ΔG, and ΔG+ . check_circle Expert Answer Want to see the step-by-step answer? See Answer Check out a sample Q&A here.
Solved Label the energy diagram for a two-step reaction ... This problem has been solved! See the answer. See the answer See the answer done loading. Label the energy diagram for a two-step reaction. Show transcribed image text.
Interpreting a Reaction Energy Diagram | Chemistry | Study.com Given the reaction energy diagram below, find the reaction energy. Step 1: Label the reactants and the products and determine their energies Remember that reactants will be on the left and products...
Solved Label the energy diagram for a two-step reaction. R Question: Label the energy diagram for a two-step reaction. R: + HX Energy RX + H RH + X Reaction coordinate Reaction coordinate Answer Bank intermediates ...
How to Draw & Label Enthalpy Diagrams - Video & Lesson ... For the following reaction: 2H_2 (g) + O_2 (g) to 2H_2O (g) Delta H = -483.6 kJ Draw the enthalpy diagram, and label the activation energy. For the following diagram, choose the correct statement.
Labeling an Energy Diagram Diagram | Quizlet Start studying Labeling an Energy Diagram. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Mechanisms and Potential Energy Diagrams | Chemistry for Non ... From the CK-12 Foundation - Christopher Auyeung. The reaction whose potential energy diagram is shown in the figure is a two-step reaction. The activation energy for each step is labeled E a1 and E a2 . Each elementary step has its own activated complex, labeled AC 1 and AC 2 .
How can I draw activation energy in a diagram? | Socratic 1. Draw and label a pair of axes. Label the vertical axis "Potential Energy" and the horizontal axis "Reaction Coordinate". 2. Draw and label two short horizontal lines to mark the energies of the reactants and products. 3. Draw the energy level diagram. There must be a hump in the curve to represent the energy level of the activated complex. 4.
Answered: Draw a reaction energy diagram for a… | bartleby Draw a reaction energy diagram for a two-step exothermic reaction whose second step is faster than the first step. Label the intermediate, transition states, ΔH, and activation energies. check_circle Expert Answer star star star star star 1 Rating Want to see the step-by-step answer? See Answer Check out a sample Q&A here.
How does the energy level diagram show this reaction is ... Label ΔH as positive or negative. Figure shows the energy level diagram for the reaction between methane and oxygen. Based on Figure, the following information can be obtained. (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. (b) During the reaction, the temperature of the mixture increases.
Solved: Draw an energy diagram for each reaction. Label ... Draw an energy diagram for each reaction. Label the axes, the starting material, product, transition state, ΔH°, and E a. a. A concerted, exothermic reaction with a low energy of activation. b. A one-step endothermic reaction with a high energy of activation. c. A two-step reaction. A → B → C, in which the relative energy of the compounds is A d.
SOLVED:Draw a reaction coordinate diagram for a two-step ... Draw a reaction coordinate diagram for a two-step reaction in which the first step is endergonic, the second step is exergonic, and the overall reaction is endergonic. Label the reactants, products, intermediates, and transition states. Answer View Answer Discussion You must be signed in to discuss. Watch More Solved Questions in Chapter 3
1. On The Energy Versus Reaction Progress Diagram Below ... 1. On The Energy Versus Reaction Progress Diagram Below, Label The Reactants And Products, Any Transition States, And Reaction Intermediates, The Change In Enthalpy For The Overall Reactions, And Any Activation Energies. Potential Energy Reaction Progress Is The Reaction Exothermic Or Endothermic?...
Energy Diagram for a Two-Step Reaction Mechanism by Ashima Singh Energy Diagram for a Two-Step Reaction Mechanism Complete Energy Diagram for Two-Step Reaction A Two-Step Reaction Mechanism The transition states are located at energy maxima. The reactive intermediate B+ is located at an energy minimum. Each step has its own delta H and














:max_bytes(150000):strip_icc()/endergonic-vs-exergonic-609258_final-2904b2c359574dfcb65a9fca2d54179a.png)

0 Response to "42 label the energy diagram for a two-step reaction."
Post a Comment