39 diagram of energy states and transitions in the hydrogen atom
The diagram represents energy levels in a hydrogen atom ... 22) The diagram represents energy levels in a hydrogen atom. The labeled transitions (A through E) represent an electron moving between energy levels. Suppose that an electron in a hydrogen atom absorbs 10.2 eV of energy, so that it moves from level 1 to level 2. What typically happens next? States of Matter - Atomic Bonding | Interaction Potential ... Watch different types of molecules form a solid, liquid, or gas. Add or remove heat and watch the phase change. Change the temperature or volume of a container and see a pressure-temperature diagram respond in real time. Relate the interaction potential to the forces between molecules.
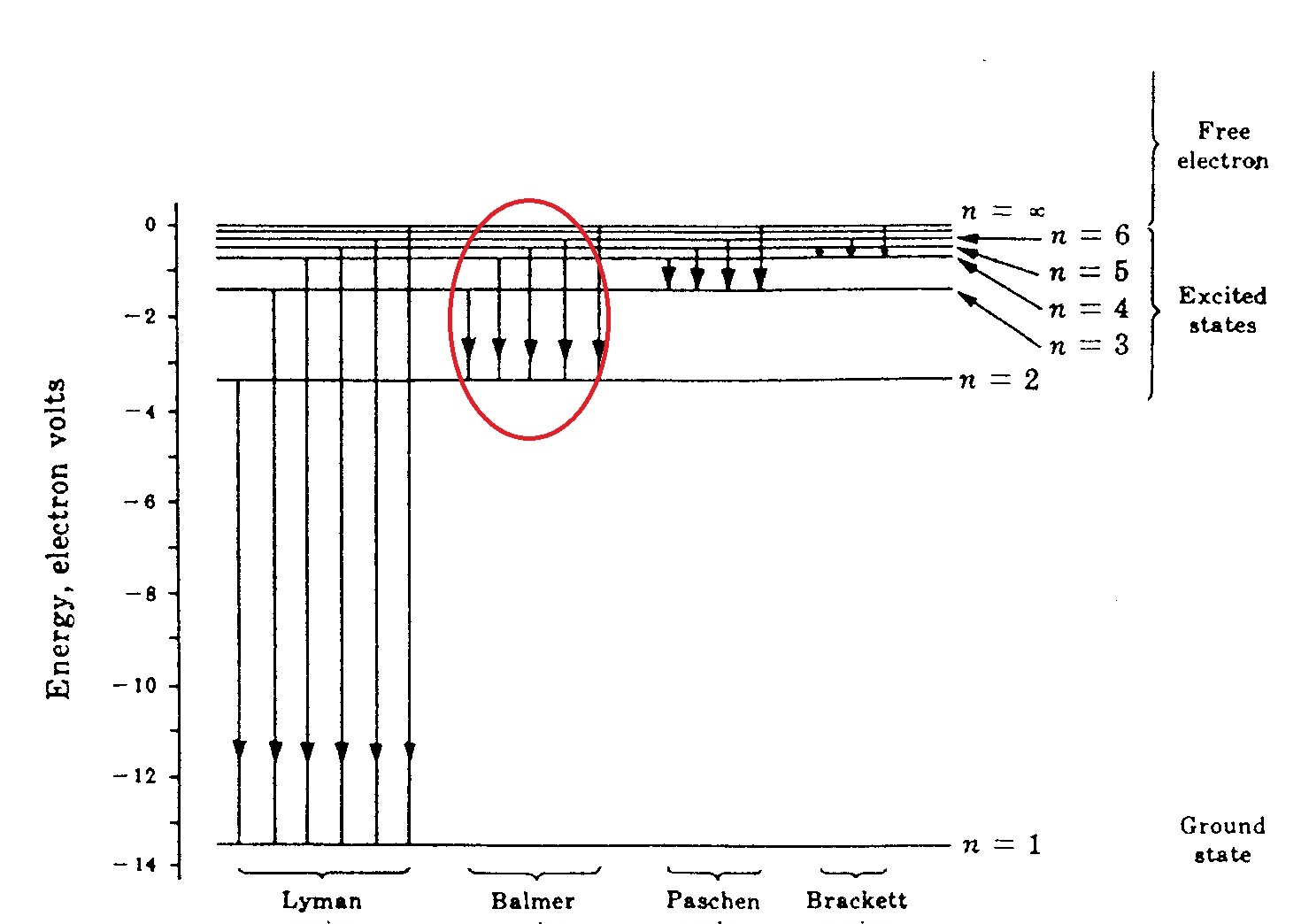
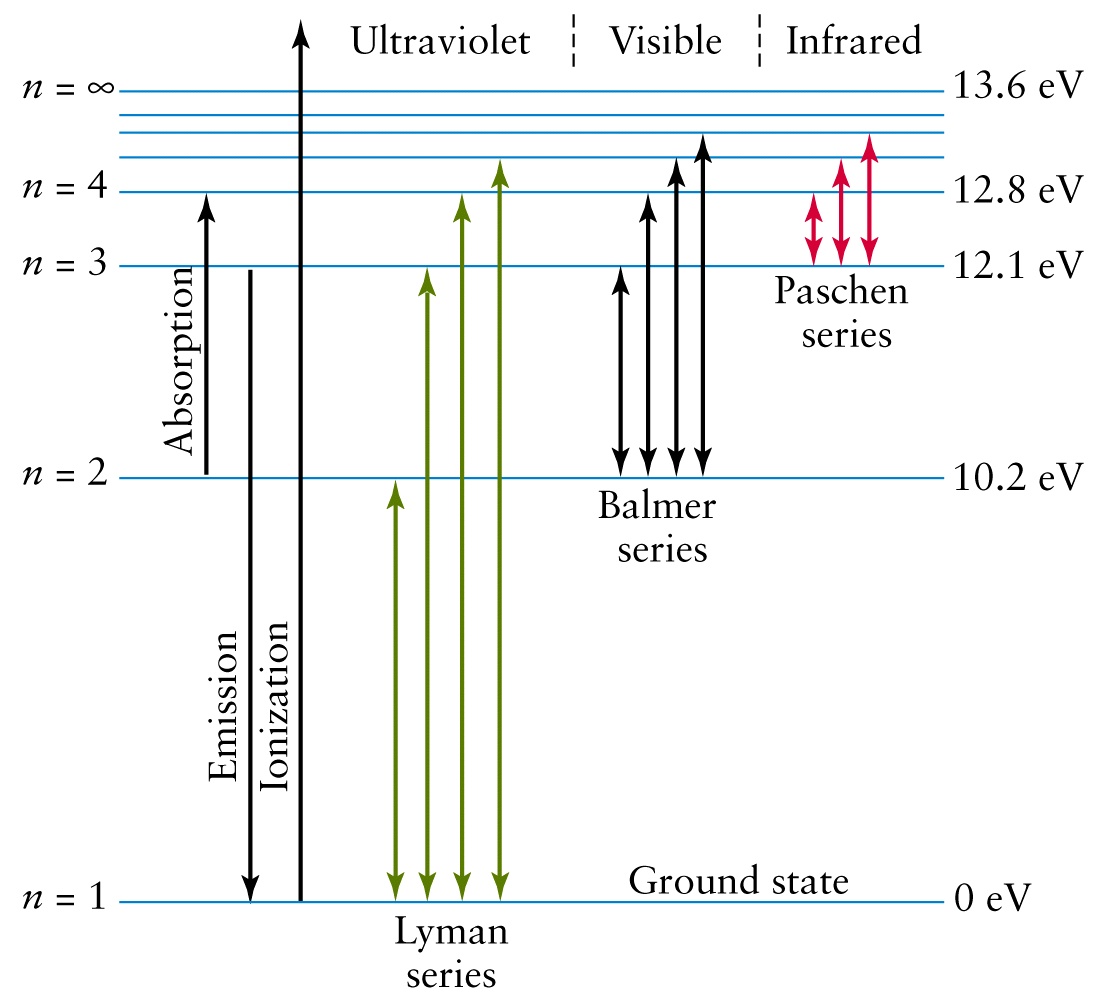
Energy Level Diagram For Hydrogen - Mini Physics The ionization energy of an atom is the energy required to remove the electron completely from the atom. (transition from ground state n = 0 to infinity n = ∞ ). For hydrogen, the ionization energy = 13.6eV When an excited electron returns to a lower level, it loses an exact amount of energy by emitting a photon.

Diagram of energy states and transitions in the hydrogen atom
Hydrogen Energy Levels - Georgia State University Electron Transitions The Bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy: This is often expressed in terms of the inverse wavelength or "wave number" as follows: The reason for the variation of R is that for hydrogen the mass of the orbiting electron is not negligible compared to ... The energy levels of an atom are as shown here. Which of ... The figure shows energy level diagram of hydrogen atom. CBSE (AIC)-2015 (i) Find out the transition which results in the emission of a photon of wavelength 496 nm (ii) Which transition corresponds to the emission of radiation of maximum wavelength ? Justify your answer. [Ans. PDF The Hydrogen atom - University of California, Irvine Hydrogen atom quantum numbers A Grotrian diagram is what spectroscopists use to analyze their line spectra. Each column is for a different l quantum number. Note that only certain transitions are observed. These are called selection rules. s p d f g h angular momentum quanta l principal quanta n
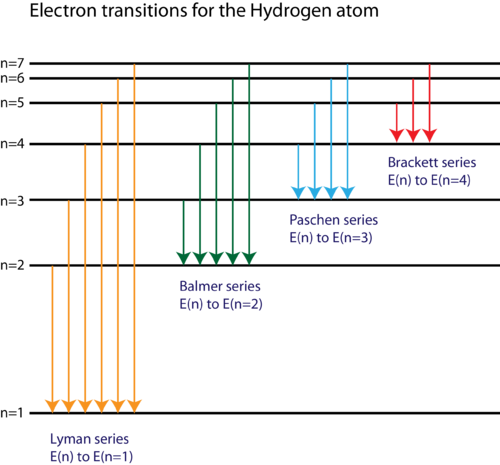
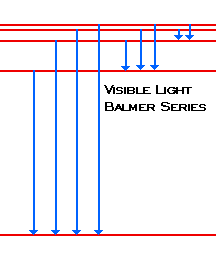
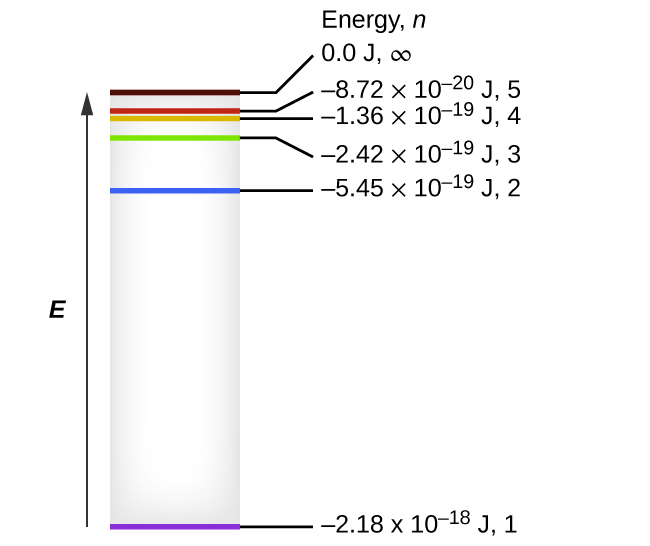
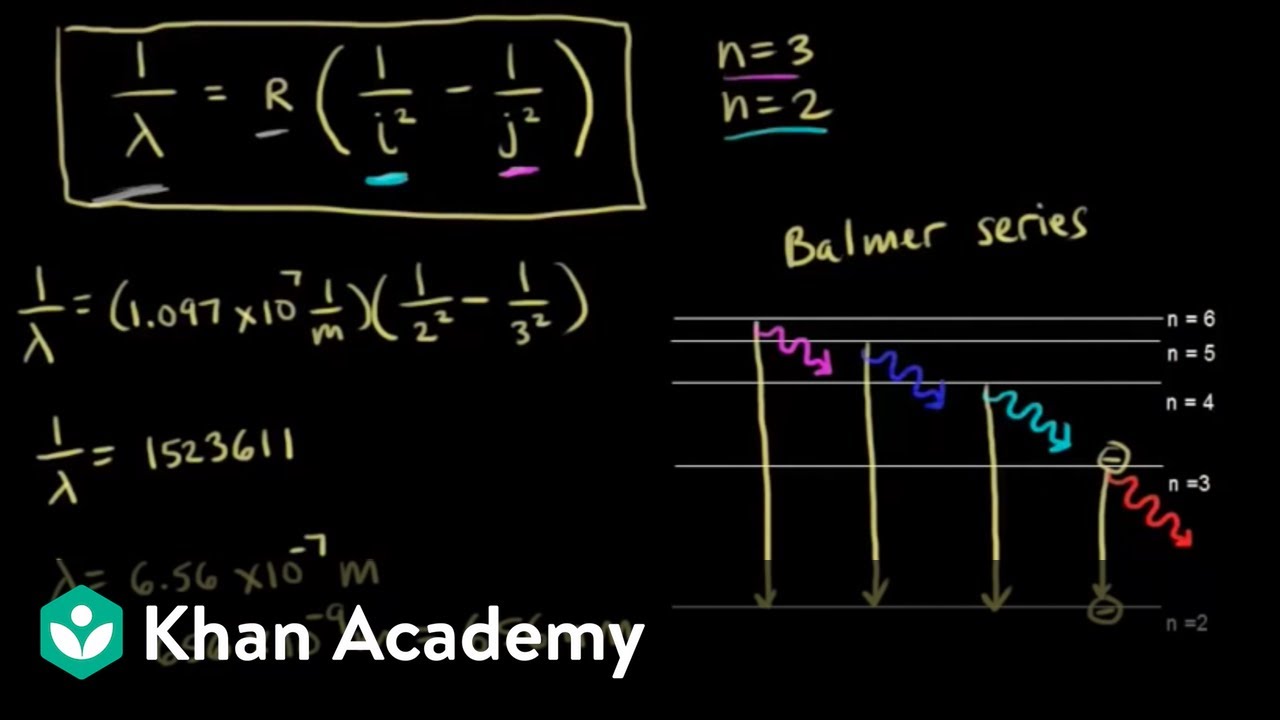
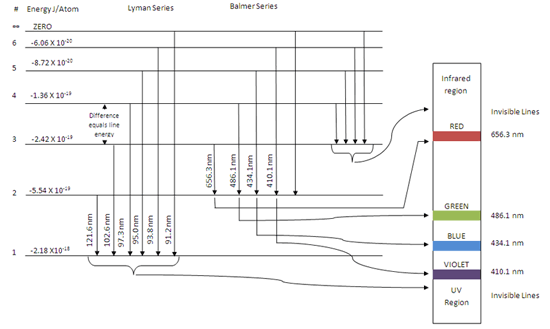
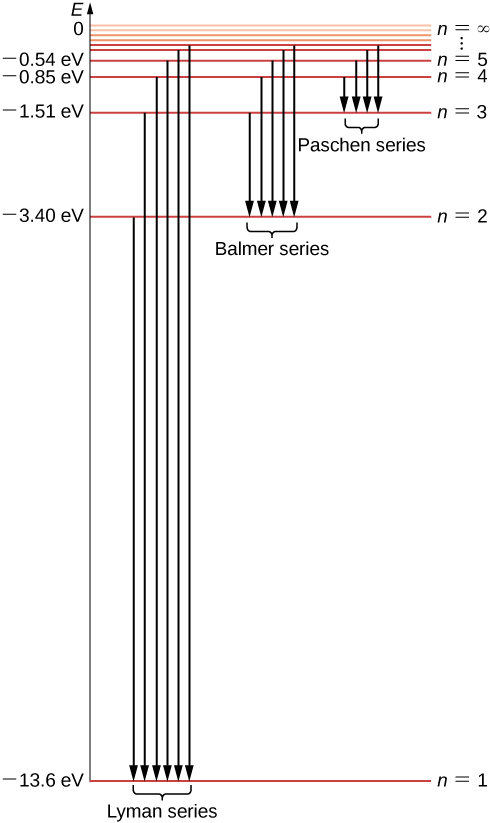
Diagram of energy states and transitions in the hydrogen atom. SOLVED:The following is an energy-level diagram for ... The following is an energy-level diagram for electronic transitions in the Bohr hydrogen atom a. Explain why the energy levels get closer together as they increase. Provide mathematical support for this. b. Verify that the colors given in the diagram are correct. Provide mathematical support. Spectral series of hydrogen atom and Energy level diagram Energy level diagram. The energy of the electron in the nth orbit of the hydrogen atom is given by, En = -13.6 /n2 eV. Energy associated with the first orbit of the hydrogen atom is, E1 = -13.6 /12 = -13.6 eV. It is called ground state energy of the hydrogen atom. byjus.com › chemistry › hydrogen-spectrumHydrogen Spectrum - Balmer Series, Definition, Diagram, Spectrum Hydrogen Spectrum - Wavelength, Diagram, Hydrogen Emission Spectrum Hydrogen Spectrum introduction We all know that electrons in an atom or a molecule absorb energy and get excited, they jump from a lower energy level to a higher energy level, and they emit radiation when they come back to their original states. Explain energy level diagram of hydrogen atom. - Sarthaks ... The total energy of an electron in its orbit for an hydrogen atom. Where 'n' is known as the principle quantum number whose value n = 1,2,3…. The energy level diagram represents different energy states with an increasing energy.
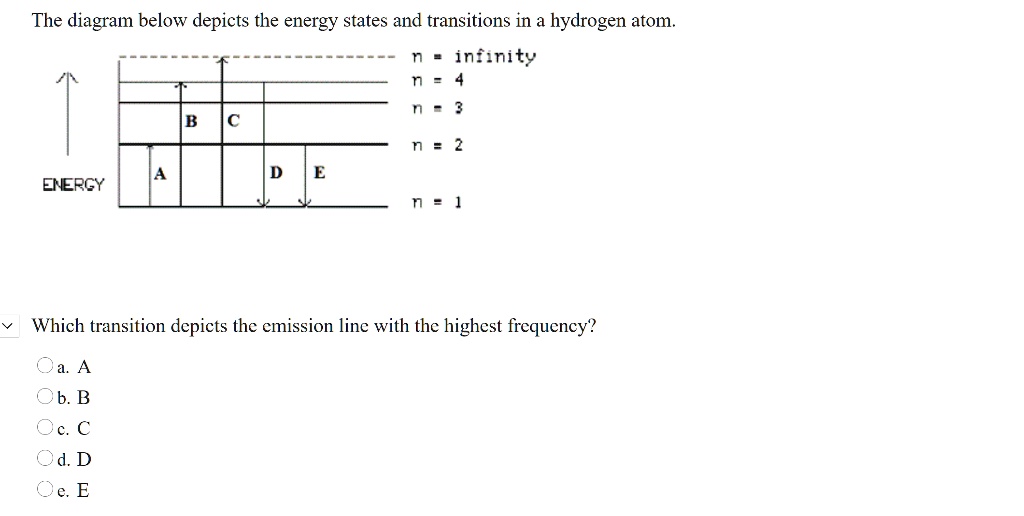
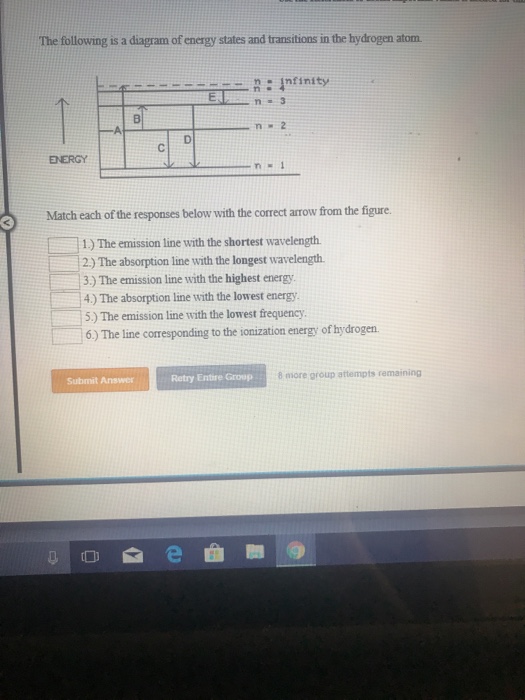
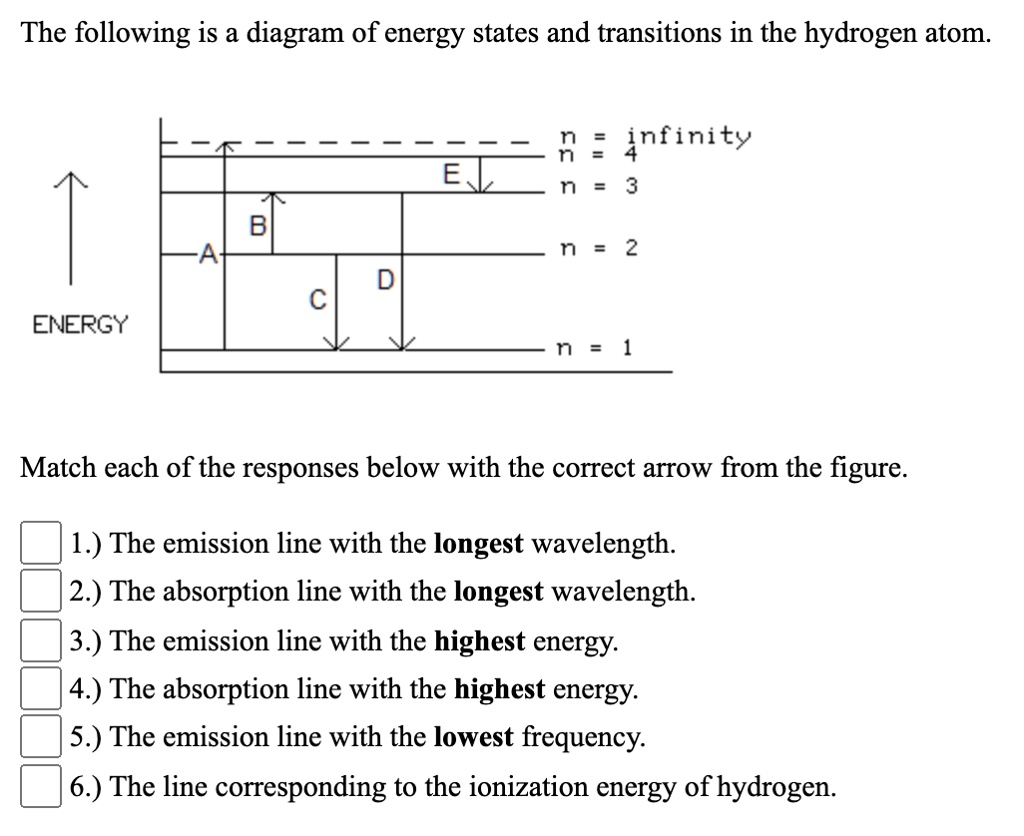
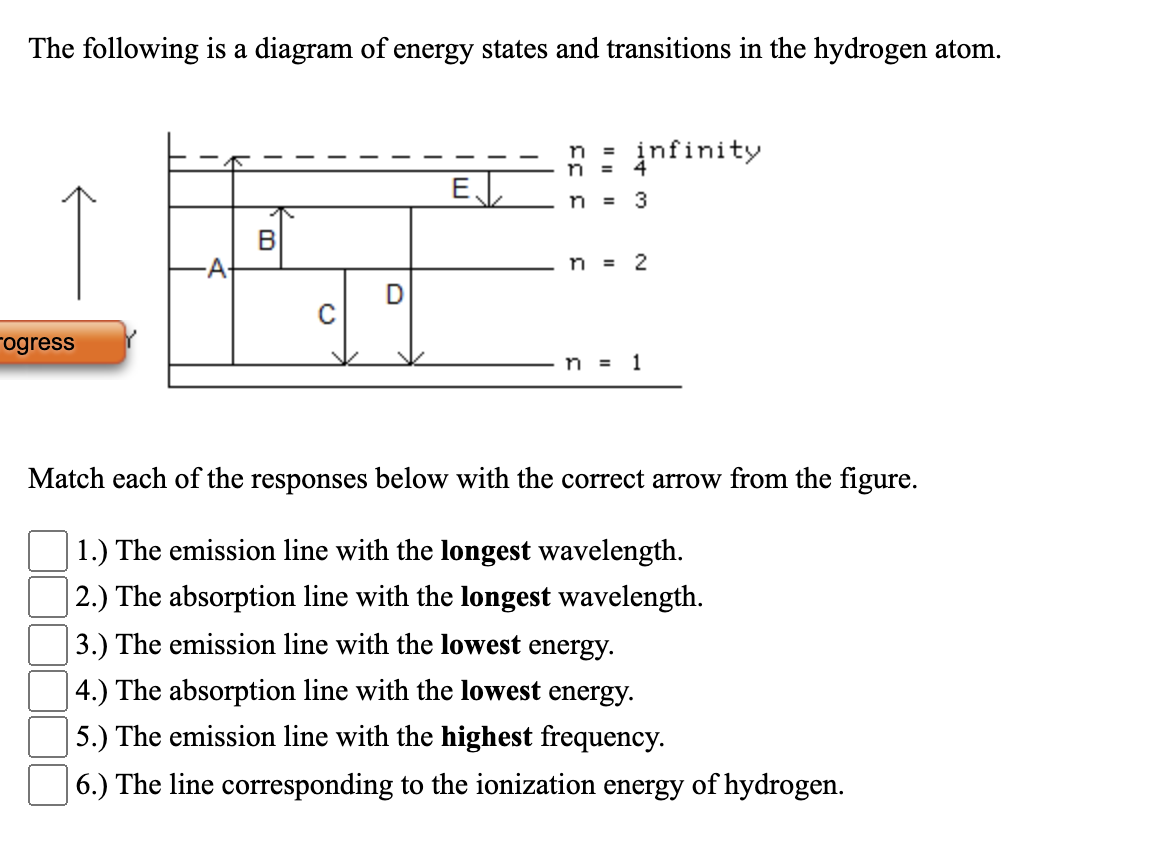
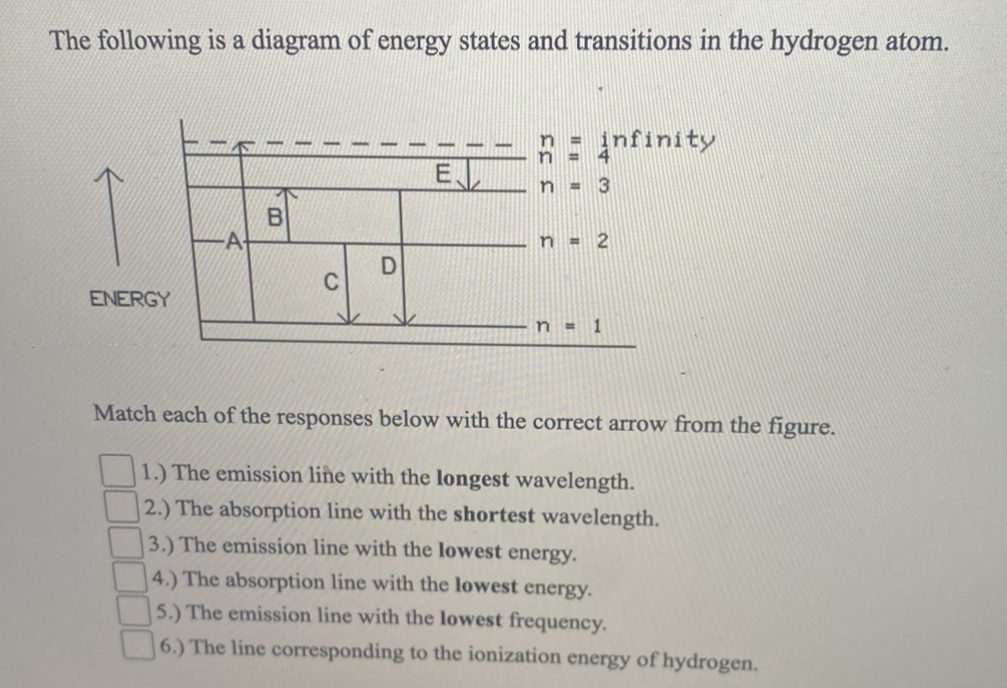
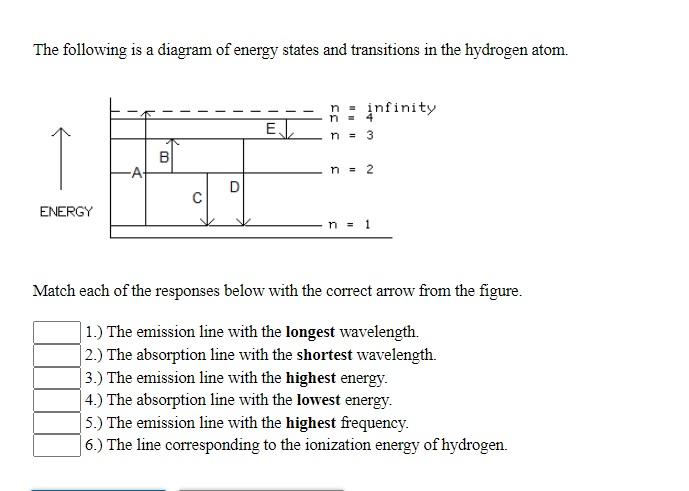
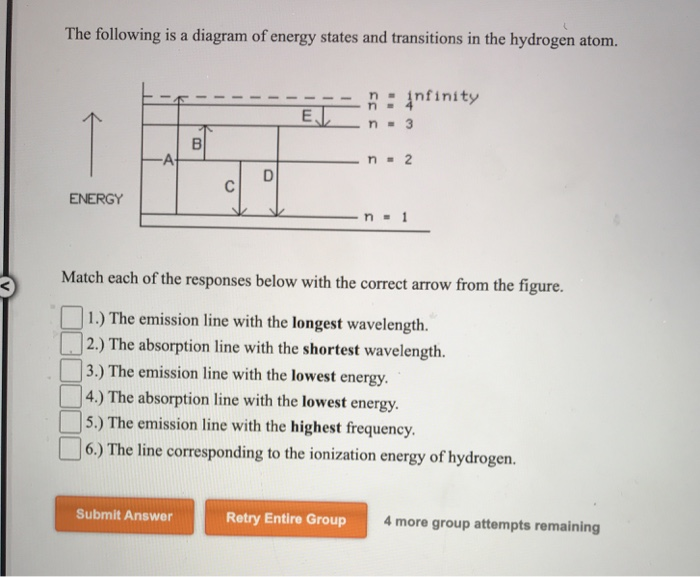
Solved The following is a diagram of energy states and ... Question: The following is a diagram of energy states and transitions in the hydrogen atom. no Infinity Cc EJ n 11 3 B -A n = 2 D C ENERGY n = 1 Match each of the responses below with the correct arrow from the figure. 1.) The emission line with the longest wavelength. 2.) The absorption line with the longest wavelength. 3.) collegedunia.com › exams › hydrogen-spectrum-seriesHydrogen Spectrum: Series, Line Spectrum Hydrogen, Wavelength Apr 13, 2022 · Whenever a hydrogen atom absorbs a photon, the electron undergoes an energy level transition, for example, n=1, n=2. When a photon passes through a hydrogen atom, the electron transitions from a higher to a lower energy state, for example, n=3, n=2. The transmission of light happens during this transition from a higher to a lower level. Hydrogen Evolution Reaction - an overview | ScienceDirect ... The α↔β transition pertains to phase transitions of the first kind. Figure 1. Schematic phase diagram of M-H system (a) and the dependence of phase transition pressure (P α↔β) on the inverse temperature (b) illustrating the hydride formation enthalpy ΔH 0 and entropy ΔS 0. In aqueous solutions, on hydrogen-sorbing materials, a potential difference is established owing … › en › explainersLesson Explainer: Electron Energy Levels | Nagwa Each of the following diagrams shows a hydrogen atom that is initially in an excited state. In each case, the electron transitions from a higher energy level to a lower energy level, emitting a photon. For which case is the energy of the emitted photon the greatest?
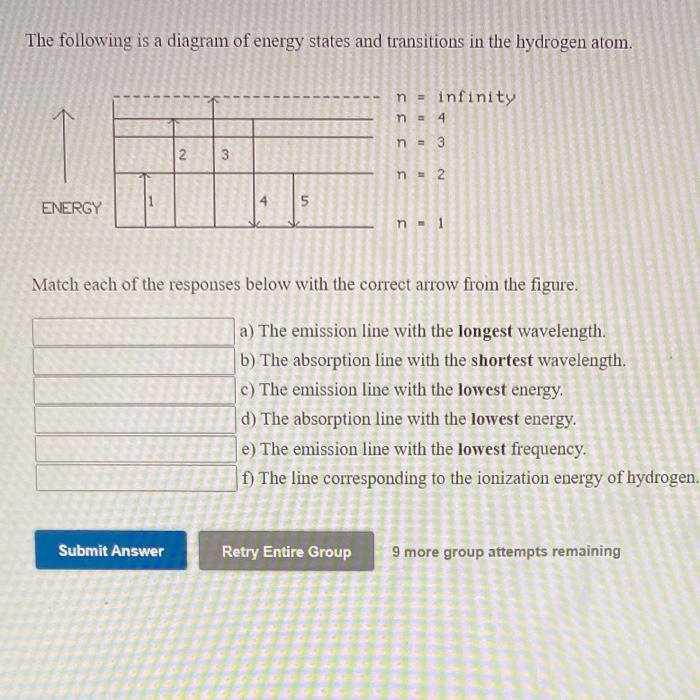
Draw an energy level diagram for hydrogen atom. Indicate ... Click here👆to get an answer to your question ️ Draw an energy level diagram for hydrogen atom. Indicate the transitions corresponding to Balmer series. The following is a diagram of energy stat... - Physical ... The following is a diagram of energy states and transitions in the hydrogen atom. - n: Infinity EL n = 3 1 B 00 -A n = 2 D C ENERGY n = 1 Match each of the responses below with the correct arrow from the figure. 1.) The emission line with the longest wavelength. 2.) The absorption line with the shortest wavelength. 3.) Draw a Neat, Labelled Energy Level Diagram for H Atom ... Solution Paschen series: i. The spectral lines of this series correspond to the transition of an electron from some higher energy state to 3rd orbit. ii. For paschen series, p = 3 and n = 4, 5,... The wave numbers and the wavelengths of the spectral lines constituting the Paschen series are given by, v ¯ = 1 λ = R ( 1 3 2 - 1 n 2) iii. The following is a diagram of energy states and | Chegg.com Question: The following is a diagram of energy states and transitions in the hydrogen atom. n = infinity n - 4 n = 3 N 3 n = 2 1 4 4 5 ENERGY n-1 Match each arrow with the correct response below. a) The emission line with the longest wavelength. b) The absorption line with the longest wavelength. c) The emission line with the lowest energy.
› III_1212 The Hyperfine Splitting in Hydrogen - The Feynman Lectures ... So if we have atoms in all four states, energy can be absorbed—or emitted—in any one of the six transitions shown by the vertical arrows in Fig. 12-4. Many of these transitions can be observed by the Rabi molecular beam technique we described in Volume II, Section 35-3 .
Solved The following is a diagram of energy states and ... The following is a diagram of energy states and transitions in the hydrogen atom. Match each arrow with the correct response below. The emission line with the longest wavelength. The absorption line with the longest wavelength. The emission line with the highest energy. The absorption line with the highest energy. The emission line with the lowest
Solved The following is a diagram of energy states and ... The following is a diagram of energy states and transitions in the hydrogen atom. n: Infinity EL n n - 3 B A n-2 D ENERGY C n = 1 Match each of the responses below with the correct arrow from the figure. 1.) The emission line with the longest wavelength. 2.) The absorption line with the longest wavelength. 3.)
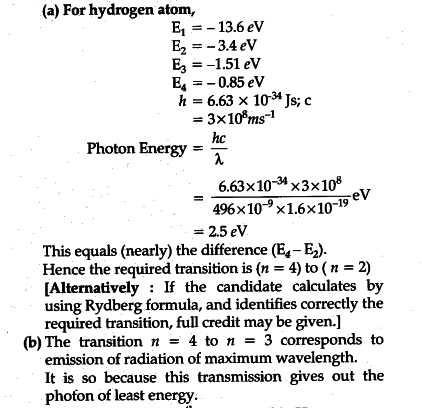
The figure shows energy level diagram of hydrogen atom. (i ... The figure shows energy level diagram of hydrogen atom. (i) Find out the transition which results in the emission of a photon of wavelength 496 nm. (ii) Which transition corresponds to the emission of radiation of maximum wavelength ? Justify your answer.
The following is a diagram of energy states and ... The following is a diagram of energy states and transitions in the hydrogen atom. Match each of the responses below with the correct arrow from the figure. a) The emission line with the shortest...
Draw a neat, labelled energy level diagram for the ... The various states arranged in order of increasing energy constitute the energy level diagram of the atom, as shown in below figure for the hydrogen atom. Here, the higher (less negative) energies are at the top while the lower (more negative) energies are toward the bottom.
Draw a neat labelled diagram showing energy levels and ... Draw a neat labelled diagram showing energy levels and transition between them for the hydrogen atoms. - Physics. ... Energy levels and transition between them for hydrogen atom: Concept: Structure of Atoms and Nuclei ... Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Commerce;
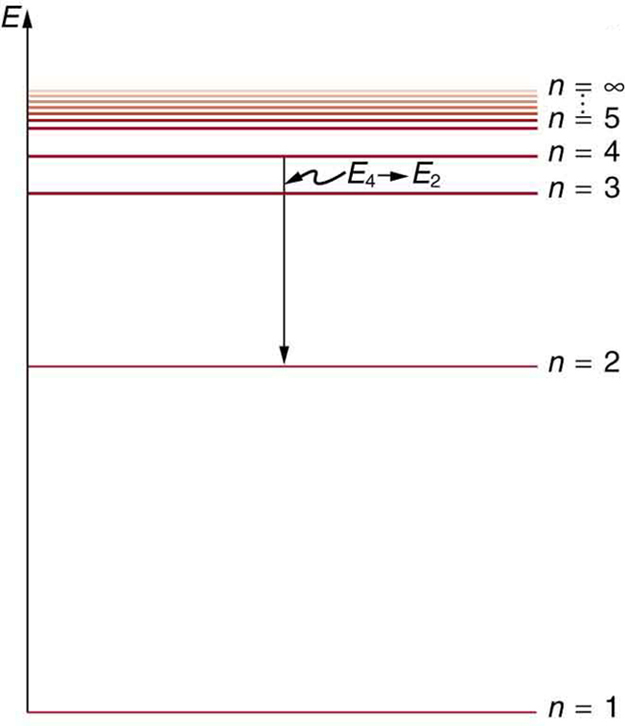
Energy level diagrams and the hydrogen atom The diagram for hydrogen is shown above. The n = 1 state is known as the ground state, while higher n states are known as excited states. If the electron in the atom makes a transition from a particular state to a lower state, it is losing energy.
en.wikipedia.org › wiki › Bohr_modelBohr model - Wikipedia The Bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. This not only involves one-electron systems such as the hydrogen atom, singly ionized helium, and doubly ionized lithium, but it includes positronium and Rydberg states of any atom where one electron is far away from everything else.
Answered: The following is a diagram of energy… | bartleby The following is a diagram of energy states and transitions in the hydrogen atom. infinity 1 3 n = 2 ENERGY Match each of the responses below with the correct arrow from the figure. 1.) The emission line with the shortest wavelength. 2.) The absorption line with the longest wavelength. 3.) The emission line with the highest energy. 4.)
Solved The following is a diagram of energy states and ... The following is a diagram of energy states and transitions in the hydrogen atom. : Anfinity FI n 1 B A n. 2 D C ENERGY Match each of the responses below with the correct arrow from the figure. 1.) The emission line with the longest wavelength. 2.) The absorption line with the shortest wavelength. 3.) The emission line with the highest energy. 4.)
courses.lumenlearning.com › physics › chapterBohr’s Theory of the Hydrogen Atom | Physics An energy-level diagram plots energy vertically and is useful in visualizing the energy states of a system and the transitions between them. This diagram is for the hydrogen-atom electrons, showing a transition between two orbits having energies E 4 and E 2 .
The Hydrogen Atom - University Physics Volume 3 The hydrogen atom is the simplest atom in nature and, therefore, a good starting point to study atoms and atomic structure. The hydrogen atom consists of a single negatively charged electron that moves about a positively charged proton ().In Bohr's model, the electron is pulled around the proton in a perfectly circular orbit by an attractive Coulomb force.
en.wikipedia.org › wiki › Energy_levelEnergy level - Wikipedia Molecules can also undergo transitions in their vibrational or rotational energy levels. Energy level transitions can also be nonradiative, meaning emission or absorption of a photon is not involved. If an atom, ion, or molecule is at the lowest possible energy level, it and its electrons are said to be in the ground state.
Transitions in Hydrogen - University of Texas at Austin Now, the energy of the eigenstate of the hydrogen atom characterized by the quantum numbers , , is , where the ground-state energy is specified in Eq. ( 678 ). Hence, the energy of the photon emitted during a transition is (1156) This corresponds to a wavelength of m. Finally, according to Eq. ( 1131 ), the transition rate is written (1157)
what is incorrect in the statement that says a hydrogen ... Find the energy (in joules) of the photon that is emitted when the electron in a hydrogen atom undergoes a transition from the n=7 energy level to produce a line in the Paschen series. Chemistry An electron jumps from energy level 1 to energy level 2 by absorbing a photon of energy 8 eV.
The following is a diagram of energy states and ... The following is a diagram of energy states and transitions in the hydrogen atom. n I infinity Match each of the responses below with the correct arrow from the figure. a) The emission line with the longest wavelength. b) The absorption line with the longest wavelength. c) The emission line with the highest energy.
Answered: The following is a diagram of energy… | bartleby Transcribed Image Text: The following is a diagram of energy states and transitions in the hydrogen atom. n = infinity n = E n = 3 B n = 2 ENERGY n = 1 Match each of the responses below with the correct arrow from the figure. 1.) The emission line with the shortest wavelength. 2.) The absorption line with the shortest wavelength. 3.)
PDF The Hydrogen atom - University of California, Irvine Hydrogen atom quantum numbers A Grotrian diagram is what spectroscopists use to analyze their line spectra. Each column is for a different l quantum number. Note that only certain transitions are observed. These are called selection rules. s p d f g h angular momentum quanta l principal quanta n
The energy levels of an atom are as shown here. Which of ... The figure shows energy level diagram of hydrogen atom. CBSE (AIC)-2015 (i) Find out the transition which results in the emission of a photon of wavelength 496 nm (ii) Which transition corresponds to the emission of radiation of maximum wavelength ? Justify your answer. [Ans.
Hydrogen Energy Levels - Georgia State University Electron Transitions The Bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy: This is often expressed in terms of the inverse wavelength or "wave number" as follows: The reason for the variation of R is that for hydrogen the mass of the orbiting electron is not negligible compared to ...













0 Response to "39 diagram of energy states and transitions in the hydrogen atom"
Post a Comment