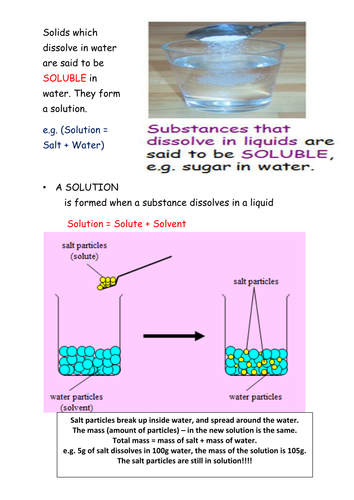
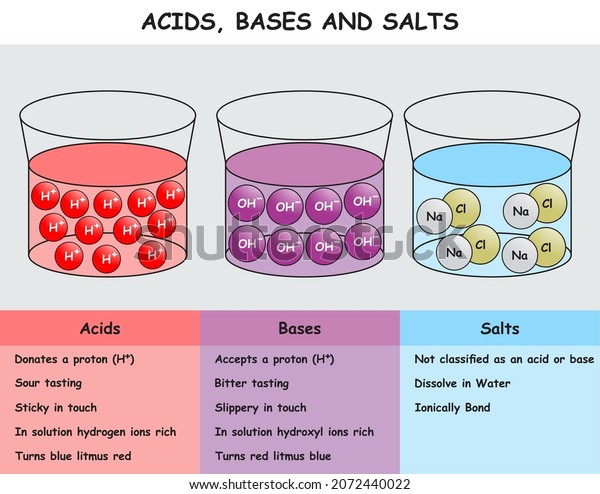
42 diagram of salt dissolving in water
Water Softener Brine Tank Level Too High Salt tank level ... Water Softener Brine Tank Level Too High Salt tank level too high, poor brine draw - diagnosis & repair. POST a QUESTION or COMMENT about how to diagnose water softener salt dosing tank or brine tank operating problems; how much water is in the brine tank, how much salt, how salty or soft is the household water, more. Why Is Dissolving Salt In Water Exothermic ... 10. Sketch the Energy Diagram (from our notes) for each of the dissolving processes. Why is dissolving Epsom salt into water endothermic? An endothermic reaction is a chemical reaction that requires heat in order to occur. … For this experiment, the Epsom salt is absorbing the heat from the water, thus causing the temperature of the water to ...
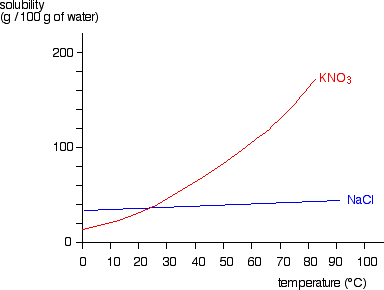
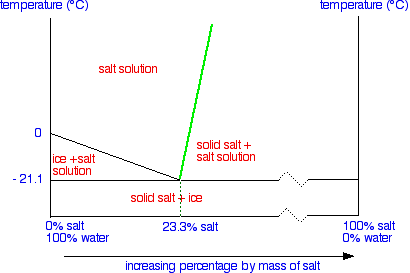
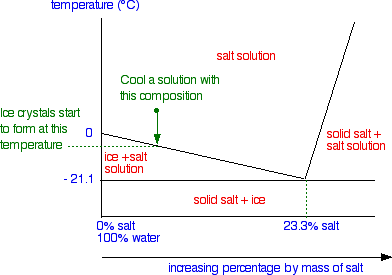
1.What is the maximum amount of the substance that will ... 1.What is the maximum amount of the substance that will dissolve in 100 3 cm of water at 30°C? A. 25 g B. 33 g C. 57 g D. 125 g 2. The graph shows the solubility curve of an unknown salt. The four points on the diagram represent four solutions of the unknown salt. Which point represents a supersaturated solution of the unknown salt? A. Q B. R ...

Diagram of salt dissolving in water
Evaporation, Distillation - Class 6, Separation of Substances This is because common salt is completely dissolve in water and not insoluble in it. We can recover common salt from salt water mixture by the process of evaporation. The solution of common salt and water is taken in a porcelain dish and heated gently by using a burner. The water present in salt solution will form water vapours and escape into ... Dissolving salt is not equivalent to applying a pressure ... Feb 10, 2022 · By advanced machine learning techniques, first-principles simulations find that dissolving salt in water does not change water structure drastically. It is contrary to the notion of “pressure ... Gummy Bears Osmosis Experiment - STEM Little Explorers Gelatine doesn't dissolve in water, but it allows water to pass through so it functions as a semipermeable membrane. Prepare your solvents. Put pure water in one glass, water with a big spoon of salt into the second glass, and vinegar into the third glass. 1 deciliter of liquid in each glass will be more than enough.
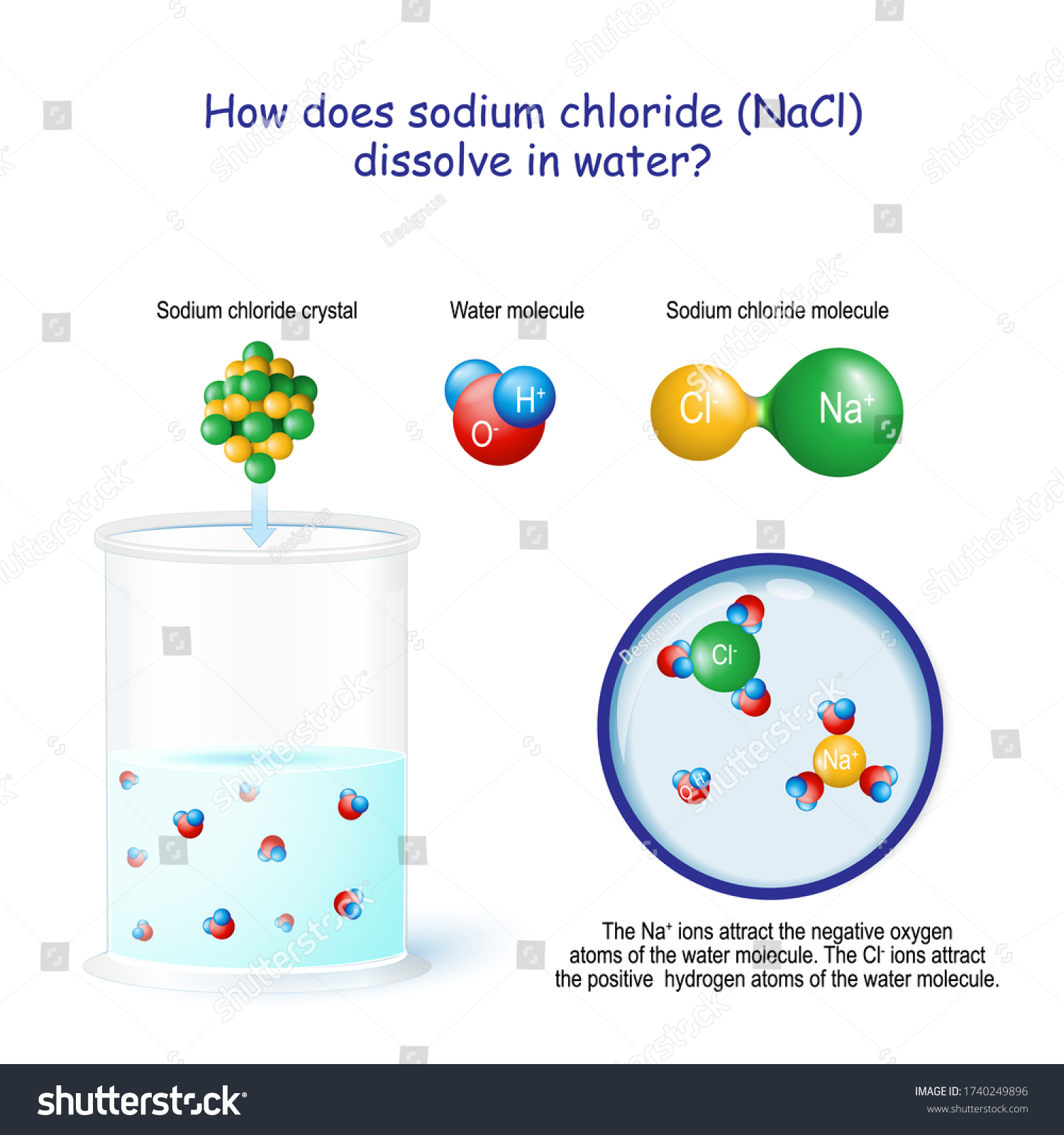
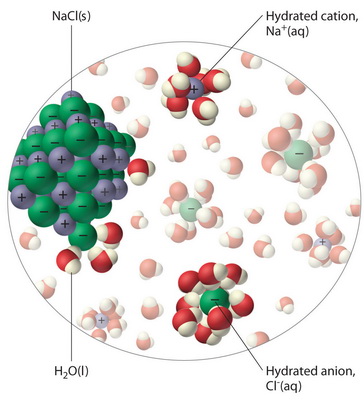
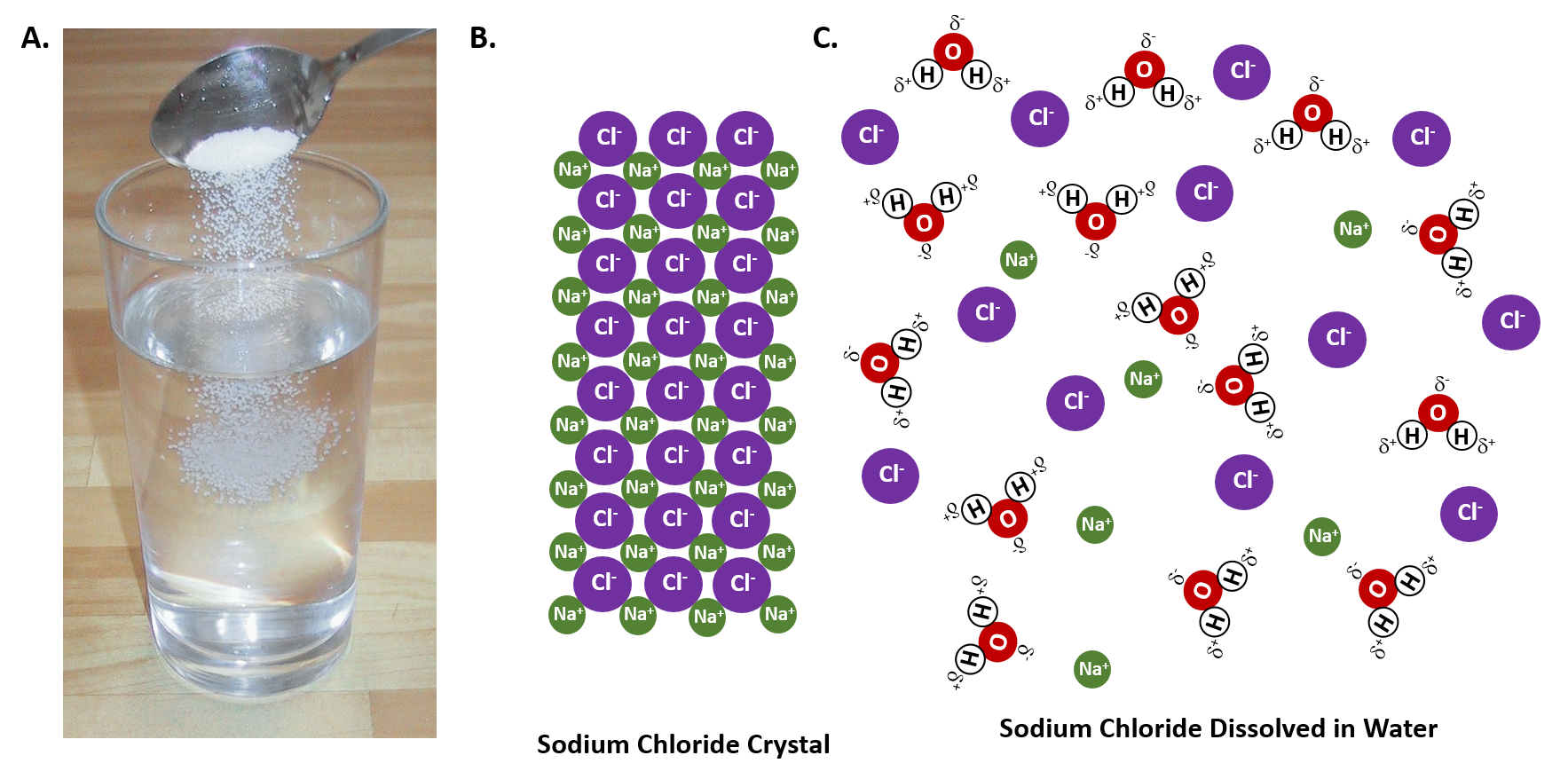
Diagram of salt dissolving in water. what happens when nacl is dissolved in water - Lisbdnet.com Explanation: Dissolving sodium chloride in water is a chemical change forming a solution of sodium and chloride ions. …. The sodium ions will not react violently with the water because the sodium is already oxidized (has lost an electron, forming a cation) as pointed out by anor77. NaCl (s) H2O⇌ Na+ (aq)+Cl− (aq) . Properties of water - Wikipedia Water has a very high specific heat capacity of 4184 J/(kg·K) at 25 °C – the second-highest among all the heteroatomic species (after ammonia), as well as a high heat of vaporization (40.65 kJ/mol or 2257 kJ/kg at the normal boiling point), both of which are a result of the extensive hydrogen bonding between its molecules. These two unusual properties allow water to … 3. Salt dissolves in water. Describe how you could obtain ... 3. Salt dissolves in water. Describe how you could obtain pure salt from a salt solution. Draw a diagram of the apparatus you would use Is Salt Water An Example Of A Homogeneous Mixture ... Salt sugar and substances dissolve in water to form homogeneous mixtures. In contrast examples of homogeneous mixtures include air salt water and steel. Now lets take the example of a saltwater sample. Examples of Homogeneous and Heterogeneous Mixtures. For example if you heat salt water the water in the solution will boil before the salt.
Is Melting Snow A Physical Change? - Knowledge Library Therefore, dissolving salt in water is a chemical change. The reactant (sodium chloride, or NaCl) is different from the products (sodium cation and chlorine anion). In contrast, dissolving a covalent compound like sugar does not result in a chemical reaction. Solubility of Common Salts: Predicting ... - Study.com Salt solubility refers to how much salt will dissolve in a specified amount of water at a particular temperature. Learn about the solubility of common salts and predicting reaction outcomes. An impure sample of table salt that weighed 0.8421 g, when ... An impure sample of table salt that weighed 0.8421 g, when dissolved in water (Question contiuned below)? September 14, 2021 thanh An impure sample of table salt that weighed 0.8421 g, when dissolved in water and treated with excess AgNO3, formed 2.044 g of AgCl. study.com › academy › answerAt 25 degrees C and 785 torr, carbon dioxide has a solubility ... Although salt is the main chemical found in ocean water, there are many others, including dissolved gases. Explore the properties of ocean water, including salinity, temperature, heat capacity ...
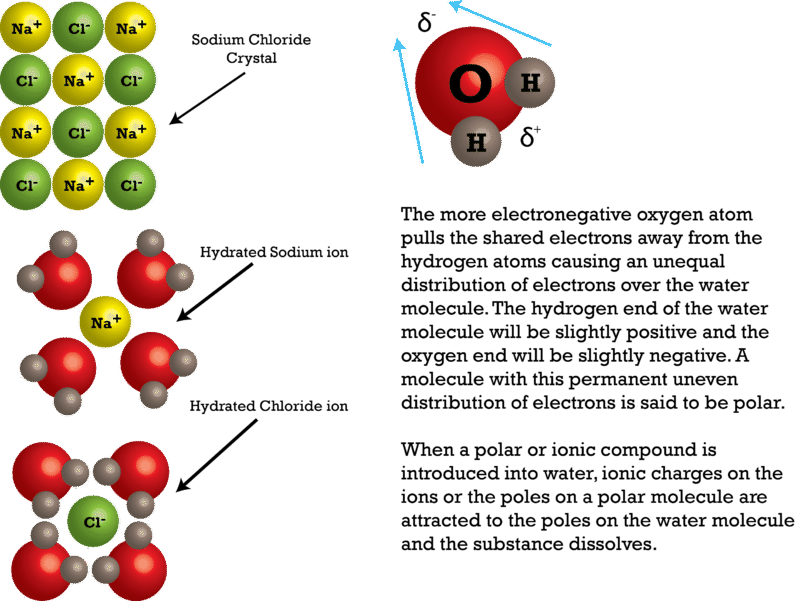
why is dissolving salt in water a physical change ... Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows.Once this happens, the salt is dissolved, resulting in a homogeneous solution. Covalent Bonding of Water (H2O) | The Ultimate Guide An ionic bond is a type of chemical bond in which the atoms have different electronegativity values from each other. For example, sodium (Na) and chlorine (Cl) form an ionic bond to make NaCl (table salt). However, in a covalent bond, the atoms are bound to share electrons. For example, if we talk about water ( H2O), it is a polar covalent bond. What happens when you put salt in apple juice? 2022 ... Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution. TYPES OF SOLUTIONS Many ionic solids dissolve in water and are called soluble salts. However, some ionic solids do not dissolve in water and do not form ions in solution. These salts are called insoluble salts and remain solid in solution. 1. Chemists use a set of solubility rules to predict whether a salt is soluble or insoluble. These rules are summarized below ...
MCQ Questions for Class 6 Science Chapter 5 ... - Learn Cram When no more salt dissolves in water at a particular temperature, then the solution at that temperature is called (a) unsaturated (b) saturated (c) super saturated (d) none of the above. Answer. Answer: (b) saturated. Question 29. The process of conversion of water into vapour is called (a) evaporation (b) condensation
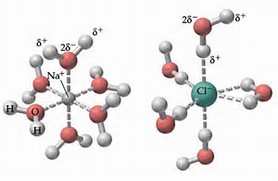
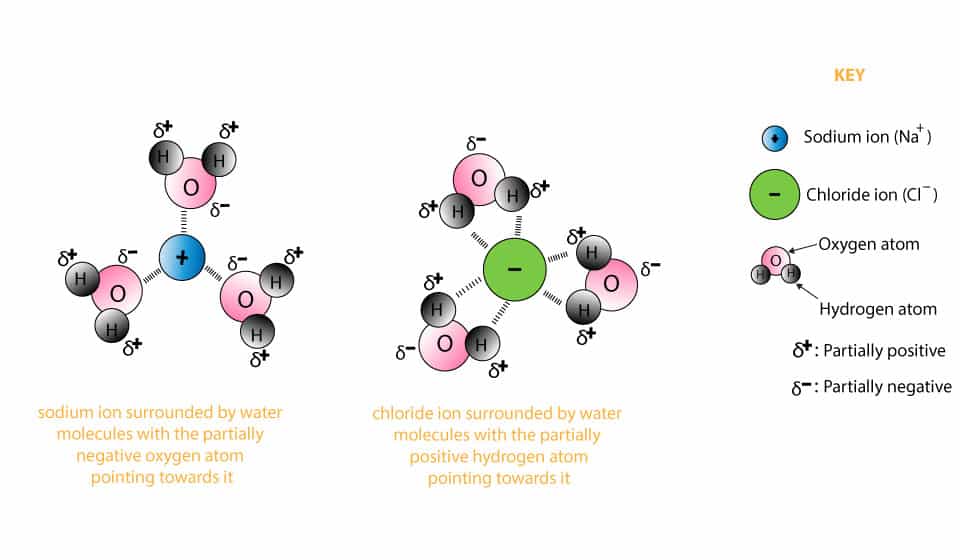
Why does table salt dissolve in water ... This diagram shows the positive and negative parts of a water molecule. It also depicts how a charge, such as on an ion Na or Cl, for example can interact with a water molecule. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative ...
Salt and Water Transport in Reverse Osmosis Membranes ... Understanding the salt–water separation mechanisms of reverse osmosis (RO) membranes is critical for the further development and optimization of RO technology. The solution-diffusion (SD) model is widely used to describe water and salt transport in RO, but it does not describe the intricate transport mechanisms of water molecules and ions through the membrane. In this …
Water-structure modifications in salt solutions are ... Salt water is ubiquitous in nature. Understanding the effect of the dissolved salts on the tetrahedral hydrogen-bond network of solvent water is essential to uncover the mechanisms underlying various physical, chemical, biological, and geological processes.
Solubility | Dornshuld Solubility indicates the amount of solute that can be dissolved in an amount of solvent under certain conditions. This is generally given as the g/100g and has the form. This can be read as "parts by weight per 100 parts by weight of solvent". However, solubilities can be given in any appropriate unit of concentration with careful conversions.
Dissolution Of Salt - science fair projects effect of ... table salt dissolves in water on make a gif. Dissolution Of Salt. Here are a number of highest rated Dissolution Of Salt pictures upon internet. We identified it from well-behaved source. Its submitted by organization in the best field. We say you will this nice of Dissolution Of Salt graphic could possibly be the most trending subject later we ...
The diagram shows abcdef all the corners of the shape are ... the diagram shows abcdef all the corners of the shape are right angles the perimeter of the shape is 28 m work out abce shown shaded on the diagram. Categories Uncategorized. Leave a Reply Cancel reply. ... A small amount of salt dissolved in water is an example of a _____.
What Happens When An Ionic Compound Such As Sodium ... Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
Water molecules and their interaction with salt ... This diagram shows the positive and negative parts of a water molecule. It also depicts how a charge, such as on an ion (Na or Cl, for example) can interact with a water molecule. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative ...
Salt can be separated from its solution salt disso - Tutorix Salt can be separated from its solution (salt dissolved in water), because (a) mixing of salt in water is a change that can be reversed by heating and melting of salt (b) mixing of salt in water is a change that cannot be reversed (c) mixing of salt in water is a permanent ' change (d) mixing of salt in water is a change that can be reversed by evaporation.
Ultrafast improvement of cellulose accessibility via non ... 29.01.2022 · Excellent dissolving ability means that MSH has great potential for improving the accessibility of cellulose. ... Recovery of lithium bromide molten salt hydrate. By evaporating the excess water under reduced pressure, ... The schematic diagram of the proposed mechanism of non-dissolving pretreatment of cellulose at room temperature.
Solvation - Wikipedia Solvation (or dissolution) describes the interaction of solvent with dissolved molecules. Both ionized and uncharged molecules interact strongly with solvent, and the strength and nature of this interaction influence many properties of the solute, including solubility, reactivity, and color, as well as influencing the properties of the solvent such as the viscosity and density.
Hydrogel: Preparation, characterization ... - ScienceDirect 01.03.2015 · Increasing water concentration has a relatively small impact on the DAA formation rate. Nevertheless, the rate roughly doubles for every 5 °C increase in temperature. For example, in an AA sample having 0.5% water, the dimerization rate is 76 and 1672 ppm/day at 20 °C and 40 °C, respectively.
Is Molten Salt Homogeneous - Universal Worksheet Our coatings significantly increase the life of critical metal parts operating in abrasive erosive corrosive and chemically aggressive environments. Molten sodium chloride for example is a liquid that can conduct electricity. One Dimensional Pseudo Homogeneous Plug Flow Reactor Model Download Scientific Diagram Electrolytes can also be smelted salts. Is molten salt homogeneous. Heavy-Water ...
What type of mixture is salt dissolved in water? - All ... Water dissolves salt because the negative part of a water molecule, the oxygen part, is attracted to the positive part of the salt, the sodium part. Is mgso4 ionic or covalent? Magnesium sulfate is an ionic compound as there is a bond between a metal and a non-metal.
What happens when salts dissociate? 2022 - Question & Answers Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
44 diagram of salt dissolved in water - Wiring Diagram Source Diagram of salt dissolved in water Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
Phase Definition and Examples - ThoughtCo 12.09.2019 · The states of matter (e.g., liquid, solid, gas) are phases, but matter can exist in different phases yet remain in the same state of matter. For example, liquid mixtures can exist in multiple phases, such as an oil phase and an aqueous phase.
Phase Diagrams – Chemistry - University of Hawaiʻi Determining the State of Water Using the phase diagram for water given in , determine the state of water at the following temperatures and pressures: (a) −10 °C and 50 kPa (b) 25 °C and 90 kPa (c) 50 °C and 40 kPa (d) 80 °C and 5 kPa (e) −10 …
How to Make Crystals From Epsom Salt - "Frosted" Windows ... When we mix the boiling water with the Epsom salt, the salt is able to dissolve and move into the empty spaces between the water molecules. See the diagram below. As the Epsom salt is stirred into the boiling water, the heated water molecules have greater space between them and the Epsom salt molecules move into the spaces.
Polarity Water Images, Stock Photos & Vectors | Shutterstock 180,338 polarity water stock photos, vectors, and illustrations are available royalty-free. See polarity water stock video clips. of 1,804. water polar molecule water molecules and ions water molecule polarity salt dissolve chemistry diagram dissolving chemistry salt in water diagram salt dissolving in water polarity of molecules water compound.
Gummy Bears Osmosis Experiment - STEM Little Explorers Gelatine doesn't dissolve in water, but it allows water to pass through so it functions as a semipermeable membrane. Prepare your solvents. Put pure water in one glass, water with a big spoon of salt into the second glass, and vinegar into the third glass. 1 deciliter of liquid in each glass will be more than enough.
Dissolving salt is not equivalent to applying a pressure ... Feb 10, 2022 · By advanced machine learning techniques, first-principles simulations find that dissolving salt in water does not change water structure drastically. It is contrary to the notion of “pressure ...
Evaporation, Distillation - Class 6, Separation of Substances This is because common salt is completely dissolve in water and not insoluble in it. We can recover common salt from salt water mixture by the process of evaporation. The solution of common salt and water is taken in a porcelain dish and heated gently by using a burner. The water present in salt solution will form water vapours and escape into ...
















![PDF] Purification and rapid dissolution of potassium sulfate ...](https://d3i71xaburhd42.cloudfront.net/8fa480682048a1b17e99415d7e26a0dfb2a0432f/5-Figure4-1.png)




0 Response to "42 diagram of salt dissolving in water"
Post a Comment