40 lewis dot diagram of ammonia
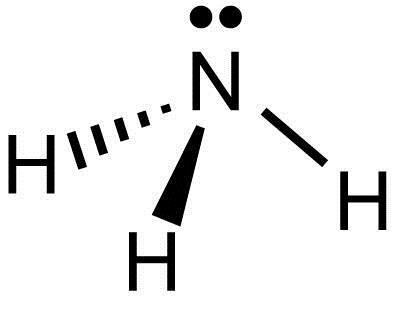
Ammonia (NH3) Lewis Structure - Steps of Drawing In the lewis structure of ammonia (NH 3), there are three N-H bonds and one lone pair on nitrogen atom. Lewis structure of NH 3 can be drawn by starting from valence electrons of nitrogen and hydrogen atoms in several steps. Each step of drawing the lewis structure of NH 3 is explained in detail in this tutorial. What Is The Lewis Dot Structure For Ammonia? [Comprehensive ... Dec 17, 2021 · Lewis Dot Diagram Of Ammonia - schematron.org Lewis dot structure of ammonia. Alternatively a dot method can be used to draw the lewis structure of NH3. Calculate the total valence electrons in NH3 molecule. N=5,H=1x3=3 Total=8 Put Nitrogen in the center and three hydrogen atoms on the sides. Lewis Dot Structures (2): Water and Ammonia.
41 draw an electron dot diagram for ammonia - Wiring ... NH 3 Ammonia is a commonly tested Lewis structure. If the species is an ion add or subtract electrons corresponding to the charge of the ion. By Drawing an Electron Dot Diagram, Show the Lone Pair Effect Leading to the Formation of Ammonium Ion from Ammonia Gas and Hydrogen Ion. CISCE ICSE Class 10.
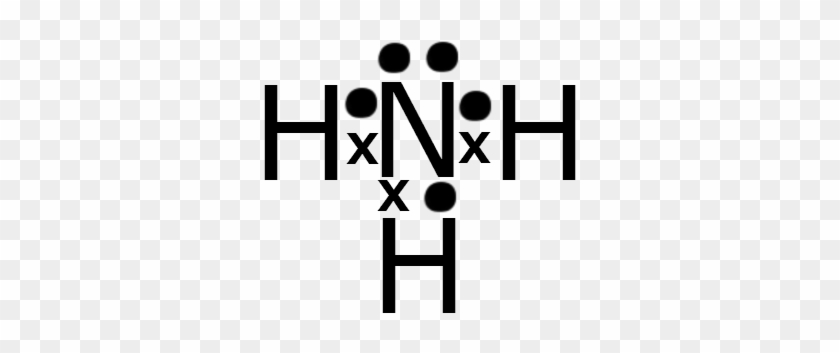
Lewis dot diagram of ammonia
Lewis Dot Diagram Of Ammonia - schematron.org Dec 27, 2018 · Lewis dot structure of ammonia. Alternatively a dot method can be used to draw the lewis structure of NH3. Calculate the total valence electrons in NH3 molecule. N=5,H=1x3=3 Total=8 Put Nitrogen in the center and three hydrogen atoms on the sides. Lewis Dot Structures (2): Water and Ammonia Drawing the Lewis Structure for NH 3. Balbharati solutions for Chemistry 11th ... - Shaalaa.com Balbharati solutions for Chemistry 11th Standard Maharashtra State Board chapter 5 (Chemical Bonding) include all questions with solution and detail explanation. This will clear students doubts about any question and improve application skills while preparing for board exams. The detailed, step-by-step solutions will help you understand the concepts better and … Draw the electron dot stucture of ammonia molecule. Draw the structure diagram of ammonia molecule as per the valence-shell electron pair repulsion theory. Hard. View solution > Draw an electron dot diagram to show the formation of ammonium ion [Atomic number: N = 7 and H = 1].
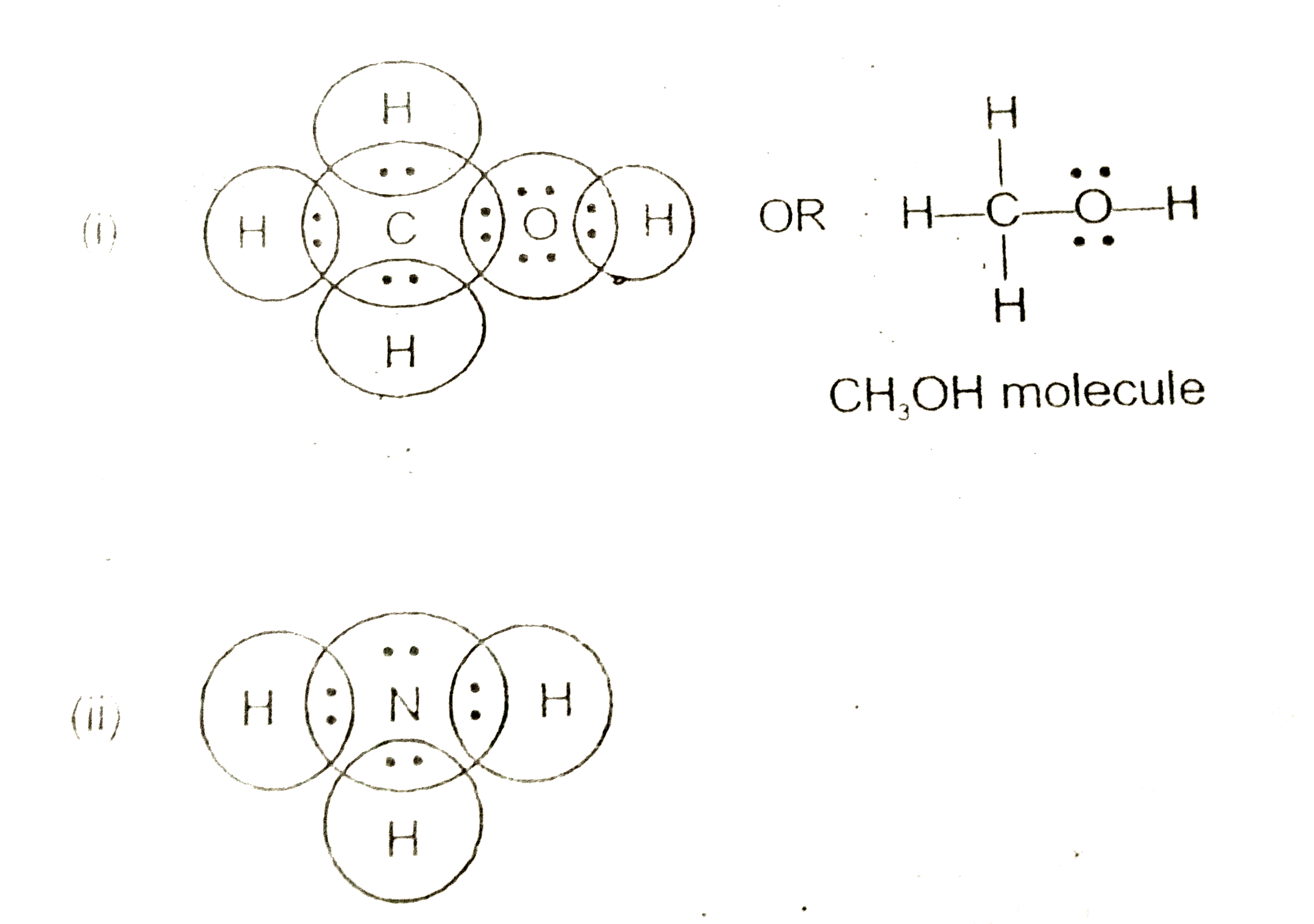
Lewis dot diagram of ammonia. The Lewis Dot Structure for NH3 - MakeTheBrainHappy The Lewis Dot Structure for NH3. Created by MakeTheBrainHappy. The Lewis Dot Structure for NH3 (Ammonia) is shown above. You could also represent the bonds as dots between the two atoms, but this may be confused with the lone pair electrons on the nitrogen. Each atom in the bond has a full valence shell, with nitrogen having access to eight ... Lewis Structure Questions and Answers | Study.com Lewis Structure Questions and Answers. Get help with your Lewis structure homework. Access the answers to hundreds of Lewis structure questions that are explained in a … NH3 Lewis Structure, Molecular Geometry, Hybridization ... Ammonia is a colorless compound, used in making fertilizers. It is a stable hydride formed of one nitrogen and three hydrogen atoms. The molecule has a pungent smell. It can form an NH4+ ion by accepting a proton. In this blog post, we will learn about the Lewis dot structure, electron geometry, and molecular geometry of this molecule. SCl2 Lewis Structure, Geometry, Hybridization, and ... 20.3.2022 · SCl2 Lewis Structure. Lewis Structure or Lewis dot structure is one of the basic methods to determine the type of bonds between atoms. In this method, electrons in the valence shell are represented by dots, and two dots on different elements can be joined to form one bond.
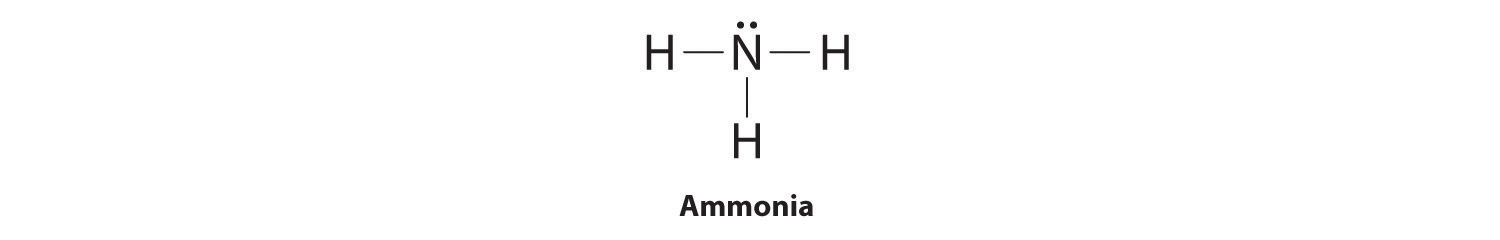
NH3 Lewis Structure - Lewis Dot Structure | Chem Helps NH3 Lewis Dot Structure To write the NH3 Lewis Structure, we need to understand the formation of NH3. The most important feature of this bond, also called electron pair bond, is that the electrons are held tightly and shared equally (jointly) by neighboring atoms. Some element atoms form a more stable structure by sharing one… Solved Draw the Lewis electron dot diagram for ammonia ... Which of the following is true regarding the Lewis diagram for ammoni a? (2 points) a. Ammonia contains three covalent bonds, one to each hydrogen atom, and the nitrogen atom has no lone pairs of electrons. b. Question: Draw the Lewis electron dot diagram for ammonia (NH3) on a sheet of scrap paper. You may consult the periodic table on Zoom to ... Rankine cycle - Wikipedia Description. The Rankine cycle closely describes the process by which steam engines commonly found in thermal power generation plants harness the thermal energy of a fuel or other heat source to generate electricity. Possible heat sources include combustion of fossil fuels such as coal, natural gas, or oil, renewable fuels like biomass or ethanol, nuclear fission, and … Lewis structure - ammonia - YouTube A simple method for drawing the Lewis structure for ammonia.
VSEPR Theory: Introduction - YouTube To see all my Chemistry videos, check outhttp://socratic.org/chemistryThis is an introduction to the basics of VSEPR Theory. VSEPR theory is a set of rules f... What is the Lewis dot diagram for nh3? - FindAnyAnswer.com Click to see full answer. Also, what is the Lewis dot structure for nh3? Explanation: The Lewis structure of ammonia, NH3 , would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of the atom. This is the reason why ammonia acts as a Lewis base, as it can donate those electrons. Lewis Dot Diagram Of Nh3 Nov 29, 2018 · Lewis Dot Diagram Of Nh3 The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons. Electron Dot Structure of NH3 by Jeff Bradbury - February 17, - Lewis Electron Dot Structure for ammonia molecule NH3. by crator-avatar Jeff Bradbury 2. 38 ammonia electron dot diagram - Wiring Diagrams Manual Lewis Dot Diagram Of Nh3 The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons. Electron Dot Structure of NH3 by Jeff Bradbury - February 17, - Lewis Electron Dot Structure for ammonia molecule NH3. The Lewis dot structure of ammonia, NH3, reveals that it ...
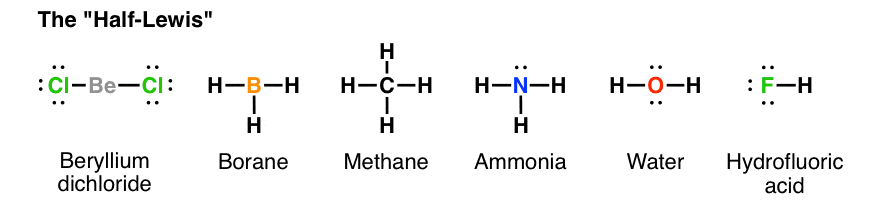
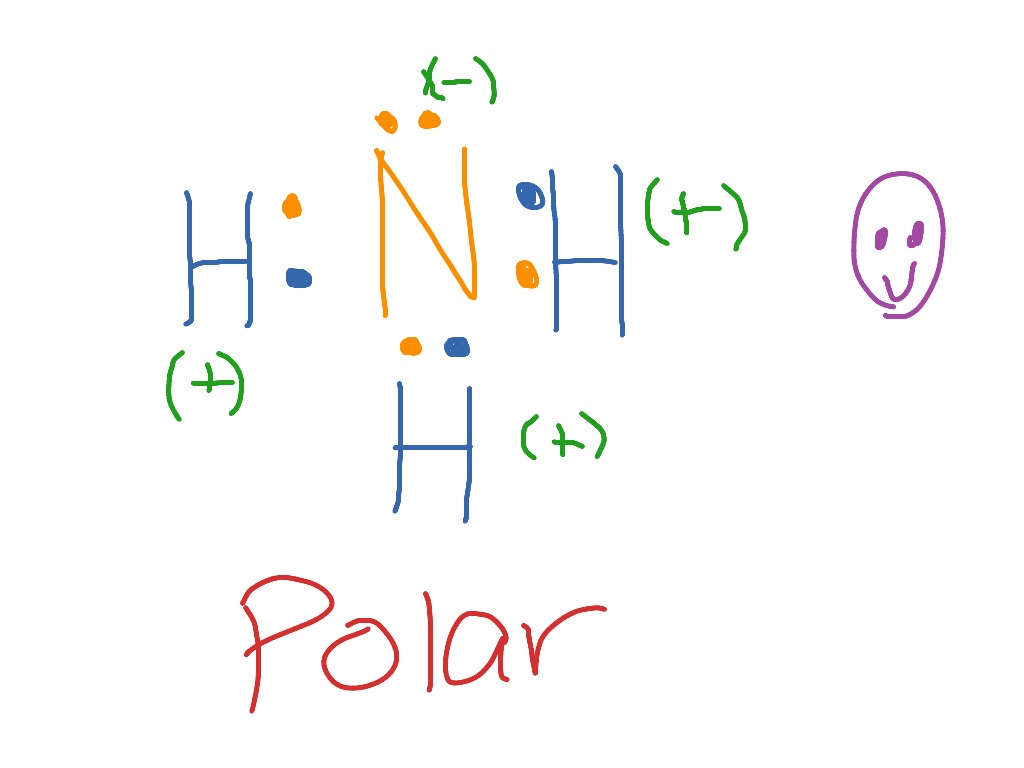
Lewis Electron Dot Structures - Detailed Explanation with ... Lewis structures, also known as electron-dot or electron-dot diagrams, are diagrams showing the bonding between a molecule's atoms and the lone pairs of electrons that may occur in the molecule. What is the Lewis structure of ammonia? Ammonia has the NH3 equation. It is extremely water-soluble because it is a polar material.
VSEPR Theory & Molecule Shapes - Video ... - Study.com 21.11.2021 · Lewis dot structure for ammonia Electron domains Number of bonding domains ... Determine the molecular shape of the molecule whose Lewis dot diagram is shown below. 3.
Lewis Dot Diagram Of Nh3 - schematron.org Mar 20, 2019 · The Lewis structure for NH3 is.The Lewis structure of ammonia, #NH_3#, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of the schematron.org is the reason why ammonia acts as a Lewis base, as it can donate those electrons.
Lewis Structure for NH3 (Ammonia) - UMD Drawing the Lewis Structure for NH 3 ( Ammmonia) Ammonia (NH 3) is a commonly tested Lewis structure due to it's widespread use in agriculture as a fertilizer. It also is a good example of a molecule with a trigonal prymidal molecular geometry. There are 8 valence electrons available for the Lewis structure for NH 3.
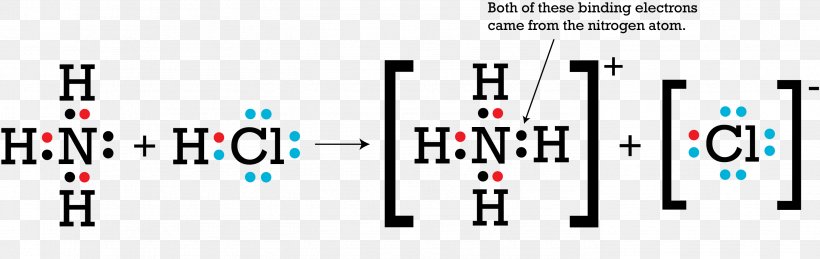
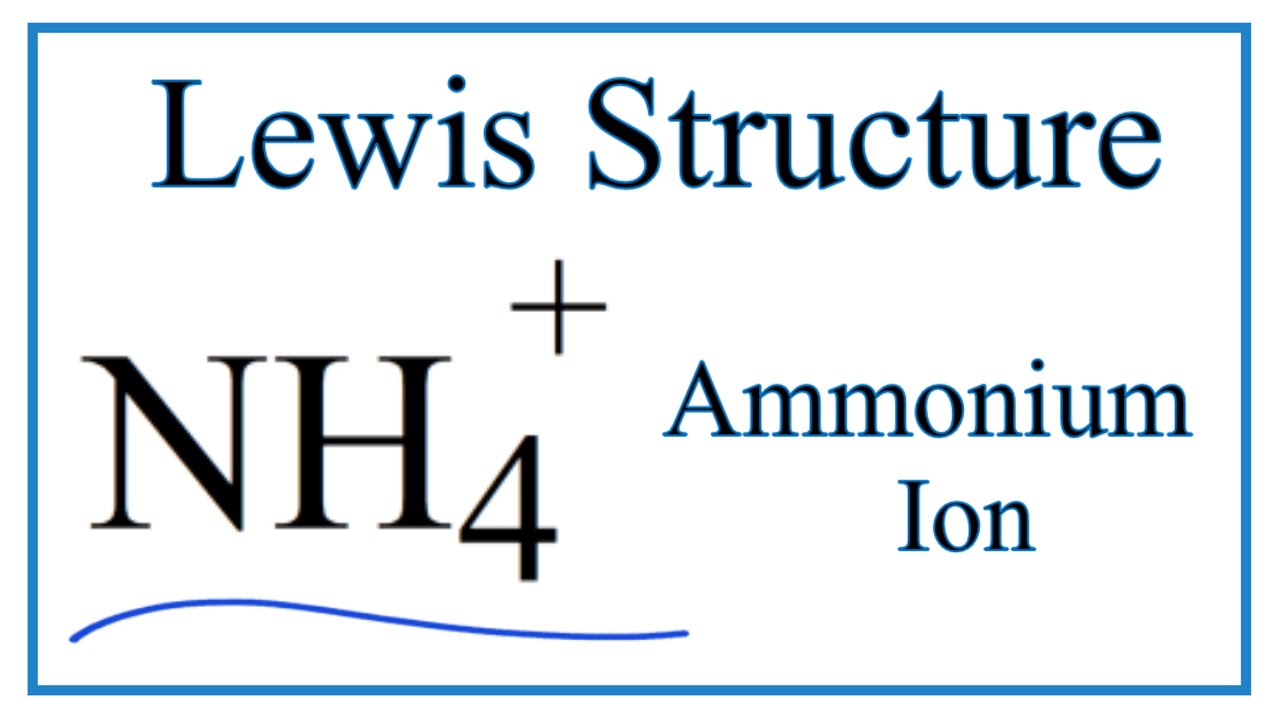
NH4+ (Ammonium ion) Lewis Structure Can I draw the NH 4 + lewis structure from NH 3 lewis structure? First, we need the reaction of forming of NH4+ ion from NH 3. NH 4 + ion is made from NH 3 and H + ion. The lone pair in NH 3 is given to the H + ion to make a new N-H bond. Now, there are four N-H bonds around nitrogen atom. See the reaction of ammonia and HCl.
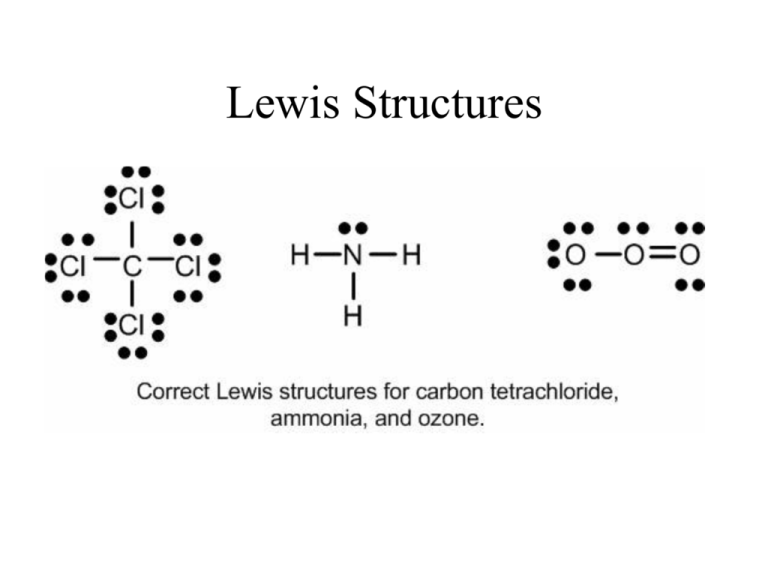
Lewis Structures: Learn How to Draw Lewis Structures ... How to Draw a Lewis Dot Structure Step 1. Determine the total number of valence electrons to be depicted in the Lewis diagram. Example: CO 2 Total = 16 Step 2. Place least electronegative element in center and draw single bonds from the central atom to other atoms. Step 3. Determine how many electrons must be added to central element.
germany-community.de 1 päivä sitten · Jan 24, 2022 · XeF2 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram. Step 1: Find the total number of valence electrons. These sp 2 hybrid orbitals lie in a plane and are directed towards the corners of an equilateral triangle …
Ammonia (NH 3 ) Lewis Structure | Steps of Drawing Ammonia lewis structure contains three N-H bonds and one lone pair on nitrogen atom. Lewis structure of NH3 can be drawn by using valence electrons of ...
Chemistry Final Review Flashcards | Quizlet The diagram shows what happens when zinc reacts with hydrochloric acid. ... The graph shows a solubility curve of ammonia gas and solubility measurements taken at different temperatures. ... Which of the following shows a correct Lewis dot structure? 5N.
The Lewis dot structure of ammonia, NH3, reveals that it ... The Lewis dot structure of ammonia, NH3, reveals that it has one lone pair of. The Lewis dot structure of ammonia, NH3, reveals that it has one lone pair of electrons and three bonds (each to a hydrogen) around the central nitrogen atom. According to VSEPR theory, what molecular shape. 13,642 results.
Lewis Structure Of Nh3 - ViralListClub.com Lewis Dot Diagram Of Nh3 The Lewis structure of ammonia NH3 would be three hydrogen atoms bonded to a nitrogen atom in the middle with a lone pair of electrons. Ammonia NH3 or H3N CID 222 - structure chemical names physical and chemical properties classification patents literature biological activities safety.
![Draw an electron dot diagram to show the formation of ammonium ion [Atomic number: N = 7 and H = 1 ].](https://d1hhj0t1vdqi7c.cloudfront.net/v1/dF9aOHFTVEFmcjA=/sd/)
Draw an electron dot diagram to show the formation of ammonium ion [Atomic number: N = 7 and H = 1 ].
By looking at the Lewis dot structure of ammonia NH3 ... The molecular weight of Ammonia is 17 g/mol. It is a colourless alkaline gas. Starting with the Lewis dot structure of Ammonia, Nitrogen has 5 valence electrons and each hydrogen has 1 valence electron. So, the total valence electrons are 8. Hydrogen always goes on the outside, so Nitrogen is the central atom.
NH3 Lewis Structure - How to Draw the Dot Structure for NH3 ... A step-by-step explanation of how to draw the NH3 Lewis Dot Structure (Ammonia).For the NH3 structure use the periodic table to find the total number of vale...
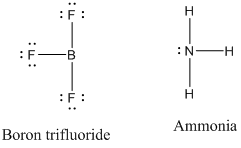
Lewis acids and bases - Wikipedia Depicting adducts. In many cases, the interaction between the Lewis base and Lewis acid in a complex is indicated by an arrow indicating the Lewis base donating electrons toward the Lewis acid using the notation of a dative bond—for example, Me3B ← NH3.Some sources indicate the Lewis base with a pair of dots (the explicit electrons being donated), which allows consistent …
Nh3 Lewis Dot Structure - ViralListClub.com Nh3 lewis dot structure. The Lewis Dot Structure for NH3 Ammonia is shown above. Formed when the atoms need to form an octet but both. NH 3 Ammonia is a commonly tested Lewis structure. If the species is an ion add or subtract electrons corresponding to the charge of the ion.
NH3 Lewis Structure, Geometry, and Hybridization ... NH3 Lewis Structure, Geometry, and Hybridization. Ammonia is the simplest binary hydride made up of nitrogen and hydrogen denoted by its chemical formulae as NH3. It is a stable pnictogen hydride where all the atoms are covalently bonded to achieve a reactive state. Ammonia is lighter than the air, colorless, and pungent in smell.
vampire-project.de 18.3.2022 · When constructing a Lewis diagram, keep in mind the octet rule, which refers to the tendency of Draw Lewis structures AND predict the molecular geometry of the following compounds or polyatomic ions: 1. youtube. JBLM Directory. PART 3: Use Lewis dot structures to show the covalent bonding in the following pairs of elements.
What is the Lewis dot structure for ammonia? – Colors-NewYork.com What is the Lewis dot structure for ammonia? In the lewis structure of ammonia (NH3), there are three N-H bonds and one lone pair on nitrogen atom. Lewis structure of NH3 can be drawn by starting from valence electrons of nitrogen and hydrogen atoms in several steps. Can you draw the electron dot structure of ammonia?
Draw the electron dot stucture of ammonia molecule. Draw the structure diagram of ammonia molecule as per the valence-shell electron pair repulsion theory. Hard. View solution > Draw an electron dot diagram to show the formation of ammonium ion [Atomic number: N = 7 and H = 1].
Balbharati solutions for Chemistry 11th ... - Shaalaa.com Balbharati solutions for Chemistry 11th Standard Maharashtra State Board chapter 5 (Chemical Bonding) include all questions with solution and detail explanation. This will clear students doubts about any question and improve application skills while preparing for board exams. The detailed, step-by-step solutions will help you understand the concepts better and …
Lewis Dot Diagram Of Ammonia - schematron.org Dec 27, 2018 · Lewis dot structure of ammonia. Alternatively a dot method can be used to draw the lewis structure of NH3. Calculate the total valence electrons in NH3 molecule. N=5,H=1x3=3 Total=8 Put Nitrogen in the center and three hydrogen atoms on the sides. Lewis Dot Structures (2): Water and Ammonia Drawing the Lewis Structure for NH 3.














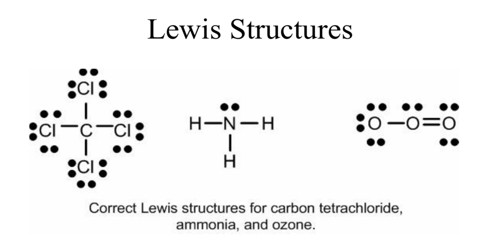






0 Response to "40 lewis dot diagram of ammonia"
Post a Comment