39 energy diagram for endothermic reaction
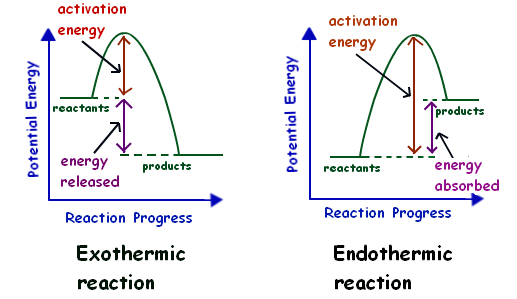
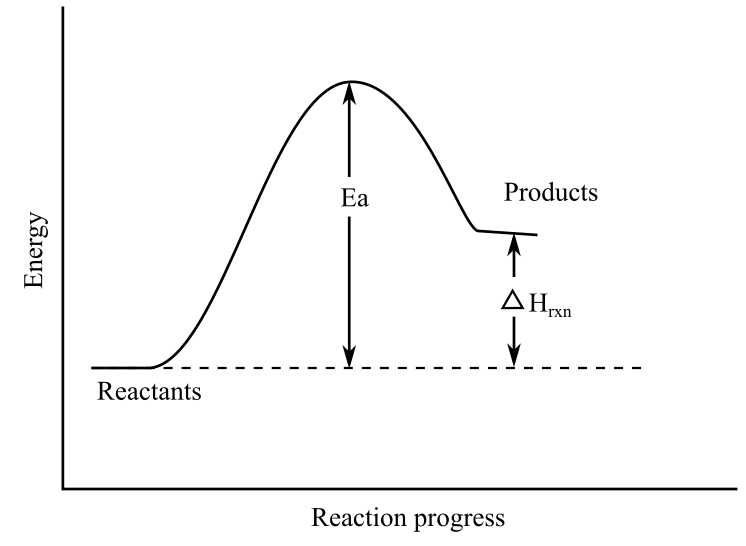
PPT Energy Diagrams: Drawing it Out Energy Diagrams: Drawing it Out Why are we learning this? Energy diagrams show how a reaction works. Energy diagrams display The energy of reactants and products The energy needed for a reaction to occur The energy absorbed or released due to reaction Energy Diagram Parts Potential Energy of the reactants Potential Energy of the products Activation Energy E - the amount of energy required to ... PDF 5.1 - Exothermic and Endothermic Reactions Enthalpy level diagrams, or energy profile diagrams, allow us to visualise what happens to the enthalpy of a reaction as it proceeds The total enthalpy of the reactant species is labelled H R , the products H P The bonds between the reactants must first be broken before they can be converted into products.
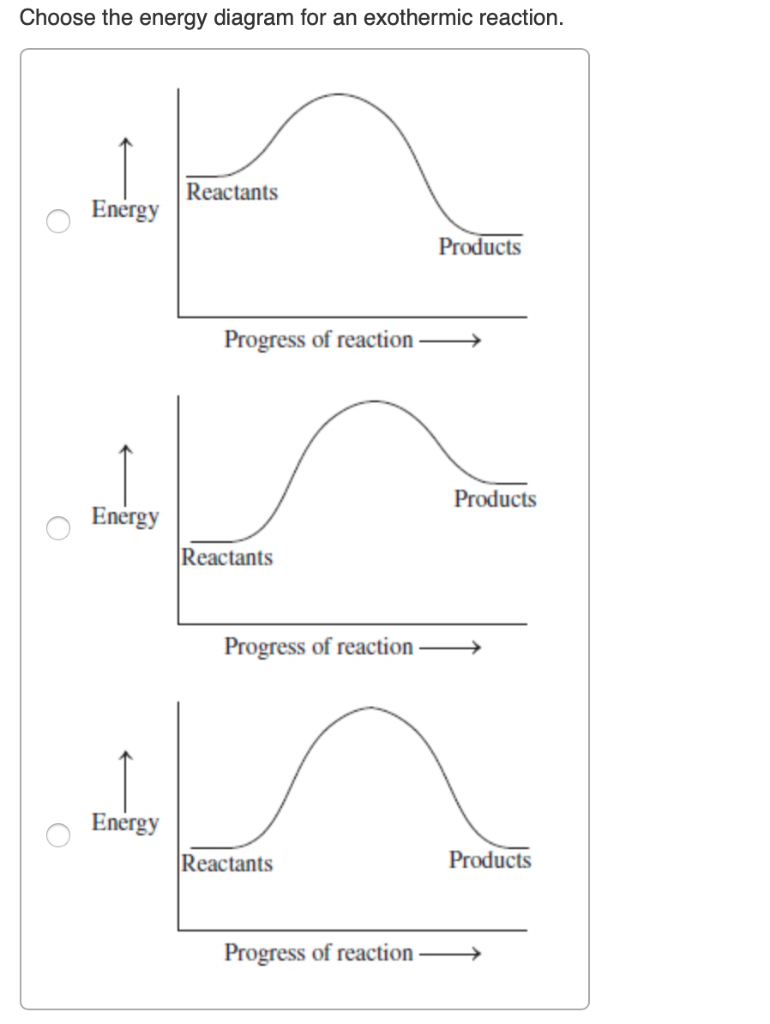
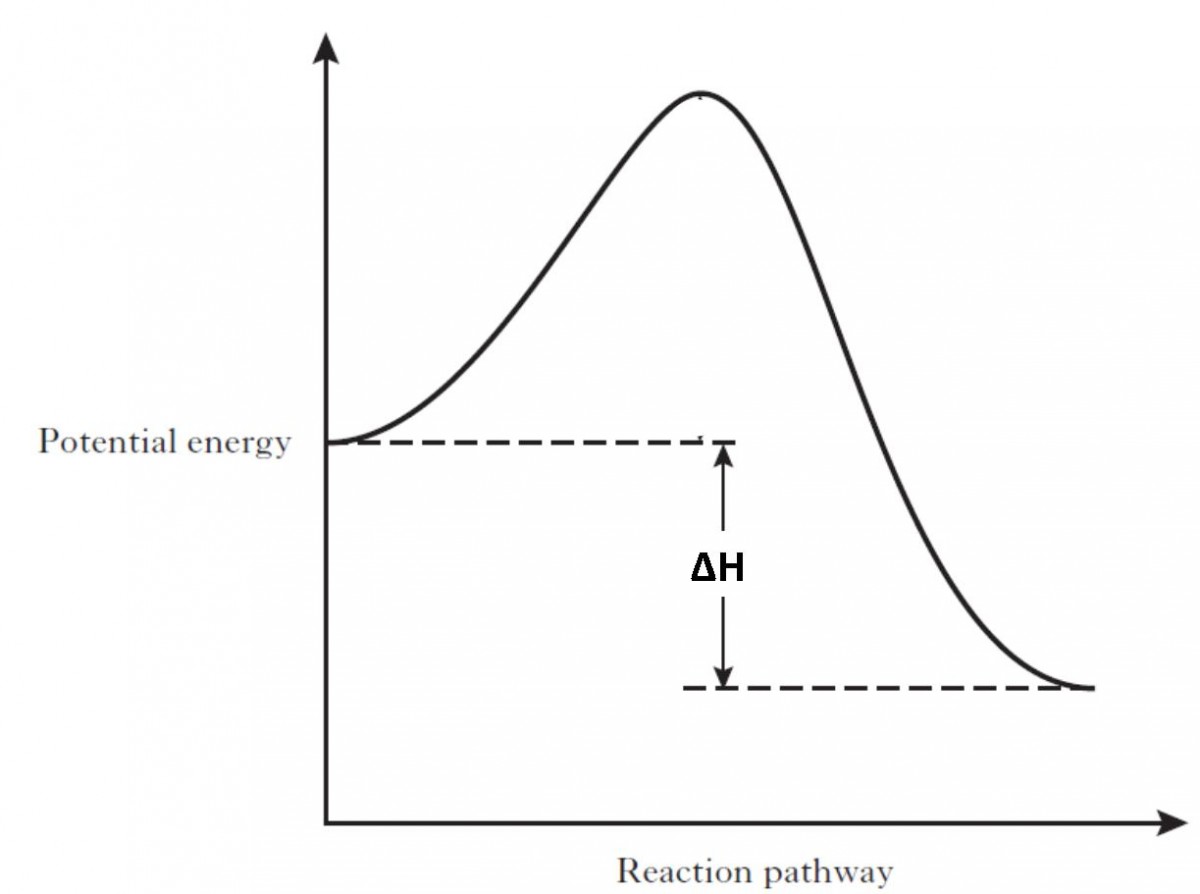
Potential Energy Diagrams | Chemistry for Non-Majors A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change is positive for an endothermic reaction and negative for an exothermic reaction.
Energy diagram for endothermic reaction
How does the energy level diagram show this reaction is ... In an endothermic reaction, the reactants absorb heat energy from the surroundings to form products. Thus, the products formed have more energy than the reactans, H products > H reactants. Therefore, ΔH is positive. Energy level diagrams are used to shows the energy content of chemicals before and after a reaction. They show: Potential Energy Diagrams - Kentchemistry.com Both endothermic and exothermic reactions require activation energy. Activated complex In this diagram, the activation energy is signified by the hump in the reaction pathway and is labeled. At the peak of the activation energy hump, the reactants are in the transition state, halfway between being reactants and forming products. Endothermic Reaction | Characteristics, Examples ... Energy level diagram for an Endothermic change. Thermal energy from the Surroundings is absorbed by the System. This results in a higher Enthalpy value of the final state with respect to the...
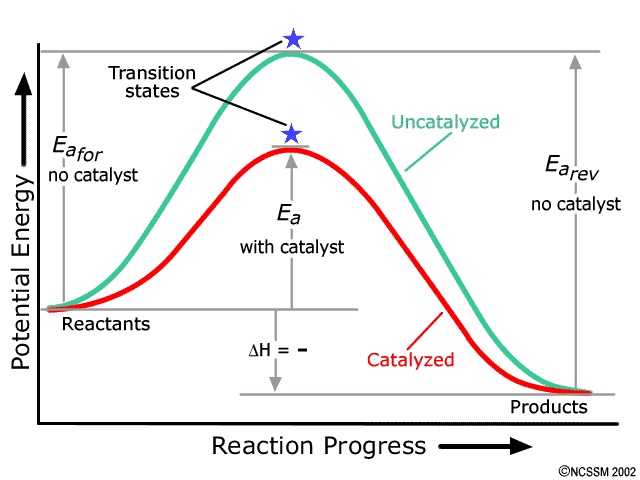
Energy diagram for endothermic reaction. Solved 15. Draw an energy diagram for a concerted, | Chegg.com 15. Draw an energy diagram for a concerted, exothermic reaction. 16. Draw an energy diagram for a two-step, endothermic reaction. 17. Rank the following carbocations in increasing stability 18. Label the reaction coordinate diagram below, providing the intermediate, reactant, product, activation energy, and transition state. B. Energy Diagrams of Reactions | Fiveable Endothermic Reactions In the graph for an endothermic reaction, you can see that the products have a higher potential energy, implying that energy has been put into the system. This further proves that ΔH is positive for an endothermic reaction. Image Courtesy of SilaVula Example PPTX Energy and Chemical Reactions - Boyertown Area School ... Endothermic Diagram. Energy absorbed in reaction. Activation . Energy. Energy used in bond. breaking. Endothermic - more energy is taken in to break the bonds in the reactants than released by the bonds being formed in the products. Therefore, energy is absorbed. Energy released in bond making Energy level diagrams - Why are there energy changes in ... The reaction shown by the second diagram is more exothermic. There is a greater difference in energy between the reactants and products. The green arrow is longer.
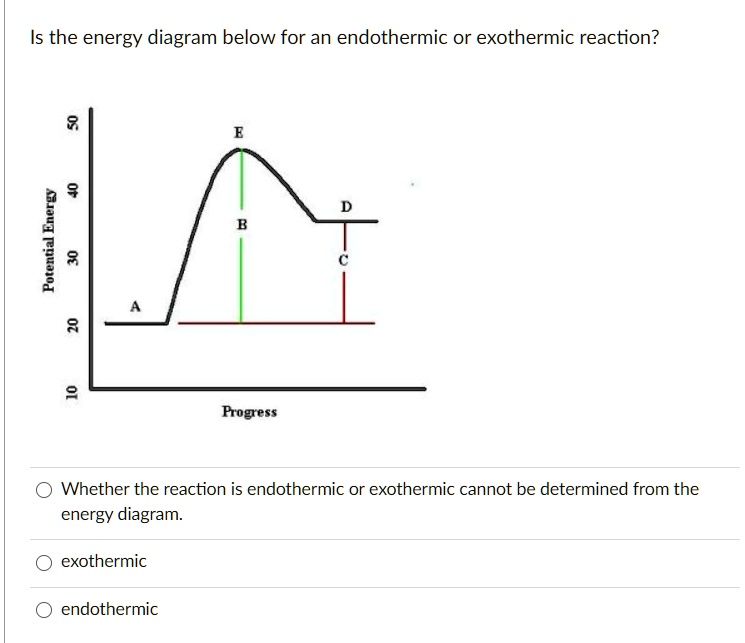
PDF Exothermic vs endothermic reaction graphs State one reason, in terms of energy, to support your answer.Answer-->Endothermic, the products have more energy than the reactants.b) On the diagram provided in your answer booklet, draw a dashed line to indicate a potential energy curve for the reaction if a catalyst is added.Answer-- 53 1 point Does this energy diagram represent an | Chegg.com Transcribed image text: 53 1 point Does this energy diagram represent an endothermic or an exothermic reaction? How can you tell? D A B с TERRI-HR Paragraph BIV E 12pt A. I EX A TIR 1 point Click on the letter that corresponds to the activation energy on this energy diagram 0 А B 55 TO Click on the letter that corresponds to the change in Gies Free Energy on the energy diagram D B 56 Click ... Energy Diagrams: Describing Chemical Reactions Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DGrxn, as well as DG1* and DG2* for the first and second activation energies, respectively. Label the positions corresponding to the transition states with an asterisk. Solved Which of the following energy diagrams shows a ... Which of the following energy diagrams shows a concerted endothermic reaction? free energy. kJ/mol Toitwool adolorib919 reaction coordinate reaction coordinate reaction coordinate reaction coordinate yam 2692 iw noite91 i r tobas (16.101 DA to ngiz adib919
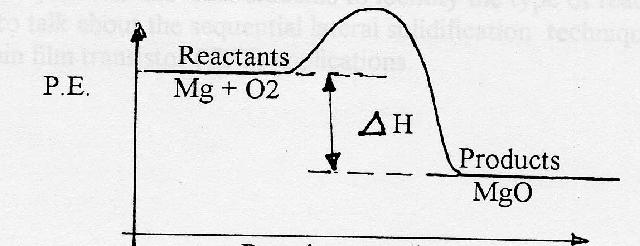
How do you know if a diagram is endothermic or exothermic ... How do you know if a diagram is endothermic or exothermic? In the energy level diagram, the enthalpies of the products are lower than that of the reactants. Hence, the enthalpy change is negative (ΔH<0). By examining this enthalpy change, one can tell whether a reaction is endothermic (ΔH>0) or exothermic (ΔH<0). How to tell if a reaction is exothermic or endothermic ... Basically, melting ice is an endothermic reaction because the ice absorbs (heat) energy, which causes a change to occur. Is Melting endothermic or exothermic? Melting is an endothermic reaction in which the total amount of heat in the substance, also known as … Endothermic Reactions: Definition, Example, Diagram and ... Endothermic Reaction Energy Level Diagram: Endothermic reactions are depicted in a basic energy level diagram below. The activation energy is the amount of energy that must be delivered to the reactants for them to break through the energy barrier and react. In an endothermic reaction, the result has higher potential energy than the reactants. PDF Potential Energy Diagrams ENDOTHERMIC OR EXOTHERMIC? Note the REACTANTS and PRODUCTS are labeled. The reactants are higher in energy (higher on y axis) then products, indicating energy is released (lost) as chemical reaction occurs, so this is an exothermic reaction. ΔH is the total energy change (enthalpy) of the reaction. ΔH is the difference in energy between the
What are Endothermic Reactions? (with Examples & Video) The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is generally lower than that of the reactant.
Energy changes - Energy changes - BBC Bitesize An energy level diagram for an endothermic reaction. In an endothermic reaction, the products are at a higher energy than the reactants. This means that the enthalpy change of the reaction (∆ H ...
Endothermic vs. exothermic reactions (article) | Khan Academy Endothermic reactions: Heat is absorbed. 1) Photosynthesis: Plants absorb heat energy from sunlight to convert carbon dioxide and water into glucose and oxygen. 6CO2 + 6 H2O + heat ---> C6H12O6 + 6O2. 2) Cooking an egg: Heat energy is absorbed from the pan to cook the egg.
12+ Endothermic Reaction Examples: Detailed Explanations Energy Diagram of an Endothermic Reaction. Image Credit: Wikimedia Commons. To know more please follow: Stereoselective vs Stereospecific: Detailed Insights and Facts. Melting of Ice to form water. Melting of ice to form water is an example of phase change reaction and it proceeds by endothermic pathway. Ice melts at 273 K or above 273K ...
Energy Diagrams | OpenOChem Learn Energy Diagrams. Exothermic versus Endothermic Reactions. Exothermic Reactions Reactions that release heat are termed exothermic. In a exothermic reaction the resulting products have more or more stable bonds than the reactants. The ΔH of reaction for an exothermic reaction is less than zero (ΔH rxn < 0).
Reaction profiles - Exothermic and endothermic reactions ... An energy level diagram shows whether a reaction is exothermic or endothermic. It shows the energy in the reactants and products, and the difference in energy between them. Exothermic reaction The...
ER10 - Temperature, Rate and Potential Energy Diagrams ... Diagram 3 - since high E a b) an endothermic reaction. Diagram 1 c) the activation energy (E a) is greater than the energy released (∆H) Diagram 3 d) a spontaneous exothermic reaction. Diagram 2 - depending on the values but looks quite small. To be spontaneous needs to be ≤ 20kJ
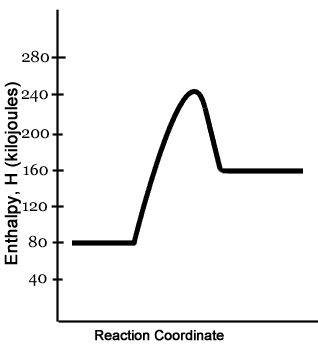
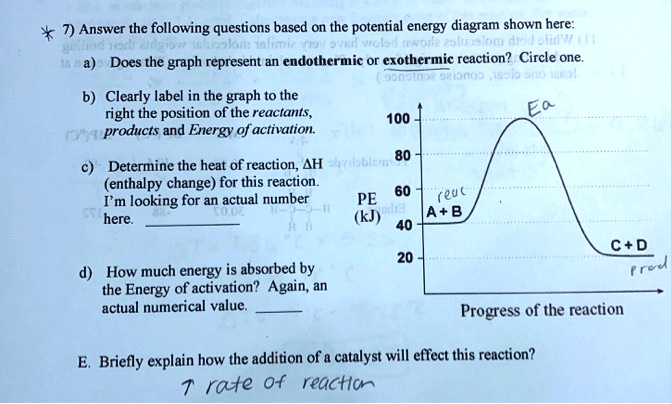
PDF Topic 5.1 Exothermic and Endothermic Reactions Heat and ... In this reaction, the total energy of the reactants is 80 kJ mol-1, the total energy of the products is -90 kJmol-1 and the activation energy for the forward reaction is 120 kJ mol-1. a) Draw a diagram of the energy profile for this reaction. Label the diagram. b) State whether the reaction is endothermic or exothermic.
The Cold Pack: A Chilly Example of an Endothermic Reaction ... Jun 01, 2020 · Energy diagrams show the energy levels of reactants and products in a reaction. General energy diagrams for exothermic and endothermic reactions (©2020 Let’s Talk Science). You can see from the diagram above that the energy level of the products of an exothermic reaction is lower than the energy level of the reactants.
Exothermic & Endothermic Reactions | Energy Foundations ... In endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are formed in the products. Endothermic reactions are accompanied by a decrease in temperature of the reaction mixture. Chris You can use energy level diagrams to visualize the energy change during a chemical reaction.
Endothermic and Exothermic reactions Flashcards | Quizlet Energy level diagram for an exothermic reaction. Energy level diagram for an endothermic reaction. freezing water into ice cubes. exothermic - HEAT LEAVES THE WATER TO FORM ICE. boiling water. endothermic - HEAT ENTERS THE WATER TO MAKE IT BOIL. evaporation.
Potential energy diagram questions chemistry pdf - Canada ... Graph 1: Use the potential energy diagram for the reaction X + Y -> Z to complete the chart below. Graph 2 1. Draw a potential energy diagram for an endothermic reaction. This is the last page. Base your answers to questions 18 through 20 on the information below.
Exhothermic and Endothermic Reactions / Activation Energy ... • In an Exhothermic reaction, the energy constantly being released by the reaction allows the process to continue once it has been started; but, in an Endothermic reaction, the process has to suck energy in continuously from the surrounding atmosphere – and by doing so, THAT is what enables it to keep going! If you were to place the liquid ...
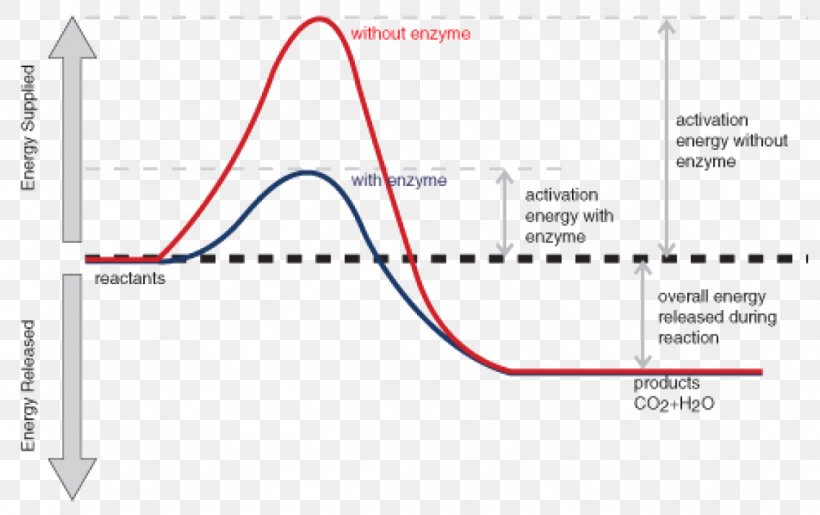
Potential Energy Diagrams - Chemistry - Catalyst ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
Exothermic and Endothermic Processes | Introduction to ... When a reaction proceeds, it either releases energy to, or absorbs energy from, its surroundings. In thermodynamics, these two types of reactions are classified as exothermic or endothermic, respectively. An easy way to remember the difference between these two reaction types is by their prefixes: endo- means to draw in, and exo- means to give off.
Endothermic and Exothermic Reactions Diagram | Quizlet Diagram of endothermic and exothermic reactions. Terms in this set (5) Exothermic Reaction. In this type of reaction, energy (in the form of heat, sound or light) is released when the reactants break apart. Heat energy can be picked up by the area surrounding the products. ... In endothermic reactions, there is less energy in the reactants than ...
Endothermic Reaction | Characteristics, Examples ... Energy level diagram for an Endothermic change. Thermal energy from the Surroundings is absorbed by the System. This results in a higher Enthalpy value of the final state with respect to the...
Potential Energy Diagrams - Kentchemistry.com Both endothermic and exothermic reactions require activation energy. Activated complex In this diagram, the activation energy is signified by the hump in the reaction pathway and is labeled. At the peak of the activation energy hump, the reactants are in the transition state, halfway between being reactants and forming products.
How does the energy level diagram show this reaction is ... In an endothermic reaction, the reactants absorb heat energy from the surroundings to form products. Thus, the products formed have more energy than the reactans, H products > H reactants. Therefore, ΔH is positive. Energy level diagrams are used to shows the energy content of chemicals before and after a reaction. They show:

















0 Response to "39 energy diagram for endothermic reaction"
Post a Comment