42 Endothermic Reaction Energy Diagram
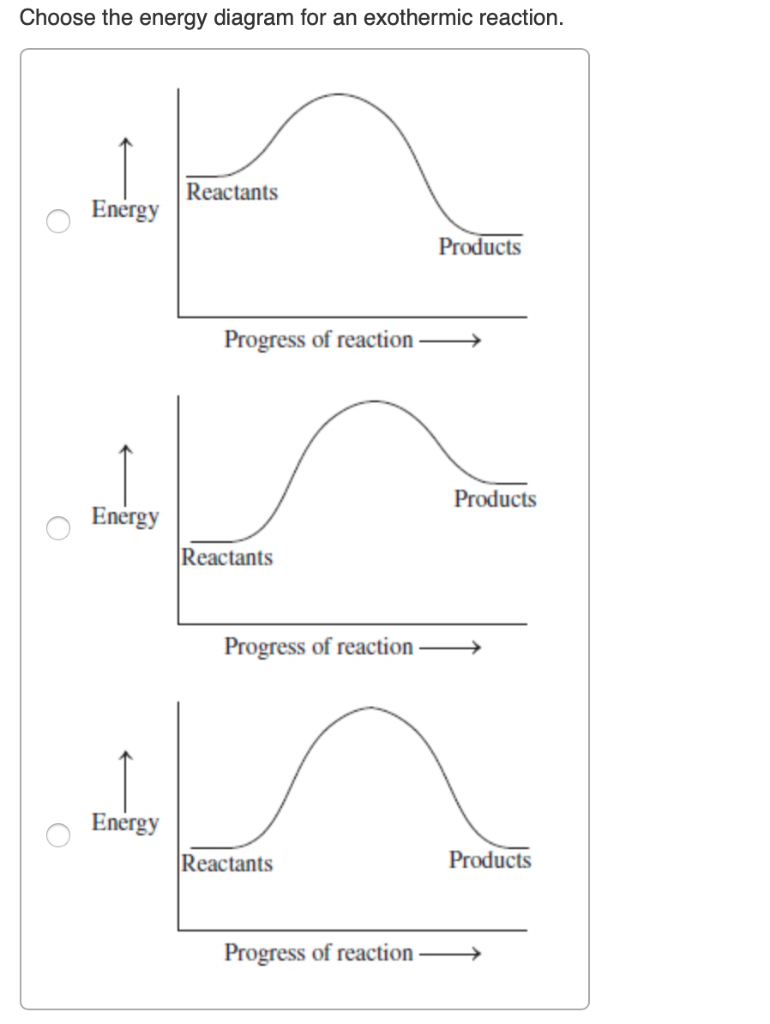
Energy Diagram — Overview & Parts - Expii Endothermic Reaction Energy Diagrams: Endothermic reactions gain energy/heat so when drawing the energy diagram, you want the reactants on the graph to be lower ... Endothermic vs Exothermic and Energy Diagrams.pdf - Name ... Task 2: Introduction to Energy Diagrams Click here to fill help you fill in this task: Endothermic vs. Exothermic and Energy Diagrams Reference Slides Energy Diagram Shows the changes in potential energy during a reaction. It starts with the _reactants_____ on the left and proceeds to the ____products_____ on the right.
PDF Endothermic and Exothermic reaction Worksheet Name Date Block Endothermic and Exothermic reaction Worksheet Name _____ Date_____ Block ____ 1 Exothermic and endothermic reactions ... An energy level diagram shows the energy taken in and released during the reaction. Add the reactants, products and their separated atoms to the correct places on the diagram.
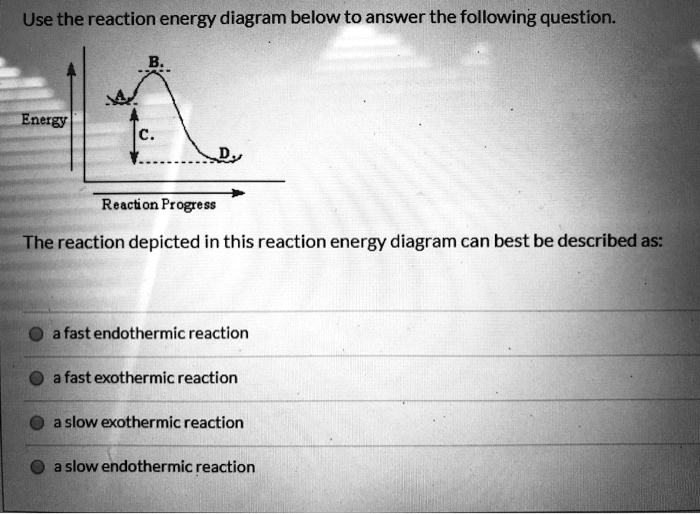
Endothermic reaction energy diagram
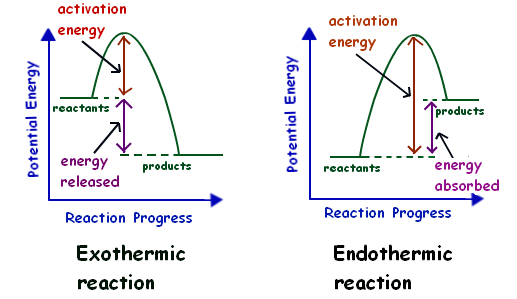
Exhothermic and Endothermic Reactions / Activation Energy ... • In an Exhothermic reaction, the energy constantly being released by the reaction allows the process to continue once it has been started; but, in an Endothermic reaction, the process has to suck energy in continuously from the surrounding atmosphere – and by doing so, THAT is what enables it to keep going! If you were to place the liquid reactants in an Endothermic reaction … PDF 5.1 - Exothermic and Endothermic Reactions 5.1.4 - Deduce, from an enthalpy level diagram, the relative stabilities of reactants and products, and the sign of the enthalpy change for the reaction Enthalpy level diagrams, or energy profile diagrams, allow us to visualise what happens to the enthalpy of a reaction as it proceeds The total enthalpy of the reactant species is labelled H R Endothermic Reaction Coordinate Diagram - schematron.org Endothermic reaction. Draw an energy coordinate diagram for both an endothermic and an exothermic reaction. In Sam's case, when ammonium nitrate was dissolved in water, the system absorbed heat from the surrounding, the flask, and thus the flask felt schematron.org is an example of an endothermic reaction.
Endothermic reaction energy diagram. What is Enthalpy? - Definition, Endothermic & Exothermic ... Enthalpy diagram. When a process begins at constant pressure, the evolved heat (either absorbed or released) equals the change in enthalpy. Enthalpy change is the sum of internal energy denoted by U and product of volume and Pressure, denoted by PV, expressed in the following manner. H=U+PV. Enthalpy is also described as a state function completely based on … Endothermic Reaction | Characteristics, Examples ... Energy level diagram for an Endothermic change. Thermal energy from the Surroundings is absorbed by the System. This results in a higher Enthalpy value of the final state with respect to the... QUESTION 2 The following reaction is endothermic | Chegg.com Transcribed image text: QUESTION 2 The following reaction is endothermic C1201268) - CH2CkC Select the energy diagram that correctly shows the relative energies of the reactants, products and the activated complex as well as the correct molecule representations of reactants products, and possible structure for the activated complex Taalt representations or reactants, products, and possible ... How to tell if a reaction is exothermic or endothermic ... The general equation for an endothermic reaction is: Reactants + Energy → Products. How can u tell if a reaction is exothermic? When a chemical reaction happens, energy is transferred to or from the surroundings. When energy is transferred to the surroundings, this is called an exothermic reaction, and the temperature of the surroundings increases. Examples of …
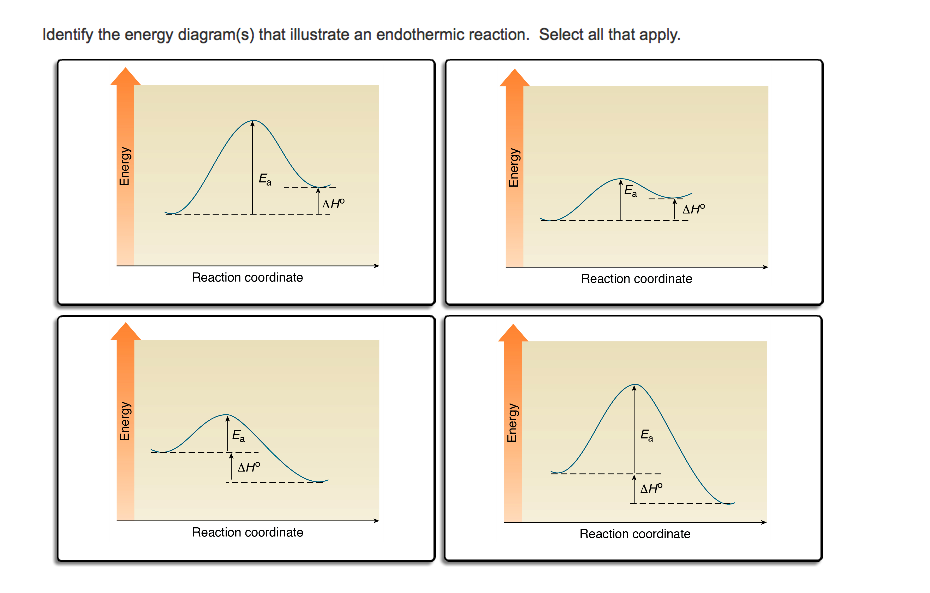
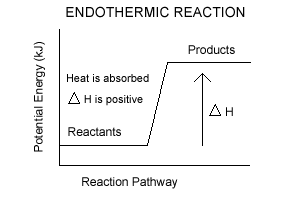
Endothermic vs. exothermic reactions (article) | Khan Academy Energy diagrams for endothermic and exothermic reactions In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other words, the products are less stable than the reactants. How does the energy level diagram show this reaction is ... In an endothermic reaction, the reactants absorb heat energy from the surroundings to form products. Thus, the products formed have more energy than the reactans, H products > H reactants. Therefore, ΔH is positive. Energy level diagrams are used to shows the energy content of chemicals before and after a reaction. They show: Endothermic and Exothermic Reactions Diagram | Quizlet Diagram of endothermic and exothermic reactions. Terms in this set (5) Exothermic Reaction. In this type of reaction, energy (in the form of heat, sound or light) is released when the reactants break apart. Heat energy can be picked up by the area surrounding the products. ... In endothermic reactions, there is less energy in the reactants than ... PPTX Energy and Chemical Reactions Endothermic Diagram. Energy absorbed in reaction. Activation . Energy. Energy used in bond. breaking. Endothermic - more energy is taken in to break the bonds in the reactants than released by the bonds being formed in the products. Therefore, energy is absorbed. Energy released in bond making
4.14 represent exothermic and endothermic reactions on a ... 4.14 represent exothermic and endothermic reactions on a simple energy level diagram. April 21, 2016 alissa 1 Comment. Activation energy: The energy needed to get the reaction started. Energy Profiles (Energy Diagrams) Chemistry Tutorial An energy profile is a diagram representing the energy changes that take place during a chemical reaction. Enthalpy change , ΔH, is the amount of energy absorbed or released by a chemical reaction. On an energy profile, the enthalpy change for the reaction is measured from the energy of the reactants to the energy of the products. PDF Representing a Reaction with a Potential Energy Diagram Representing a Reaction with a Potential Energy Diagram (Student textbook page 371) 11. Complete the following potential energy diagram by adding the following labels: an appropriate label for the x-axis and y-axis, E a(fwd), E a(rev), ΔH r. a. Is the forward reaction endothermic or exothermic? b. Reaction Coordinate Diagrams The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction. Together, the products O 2 and atomic O, have a higher energy than the reactant O 3 and energy must be added to the system for this reaction.
Endothermic Reactions: Definition, Example, Diagram and ... Sep 02, 2021 · Endothermic Reaction Energy Level Diagram: Endothermic reactions are depicted in a basic energy level diagram below. The activation energy is the amount of energy that must be delivered to the reactants for them to break through the energy barrier and react. In an endothermic reaction, the result has higher potential energy than the reactants.
Endothermic Reaction Coordinate Diagram - Wiring Diagrams Below is a reaction coordinate diagram for an endothermic reaction. A reaction coordinate diagram shows the energy changes that take place in each of the steps of the mechanism. In a reaction coordinate diagram, the total energy of all species is plotted against the progress of the reaction.
Energy Diagrams of Reactions | Fiveable Nov 23, 2021 · Enthalpy, or heat energy, is represented by ΔH (Δ is the delta sign, which means change). If there is a negative change in energy, or -ΔH, an exothermic reaction is taking place and energy is released🔥 from the system to the surroundings. If there is a positive change in energy, or +ΔH, an endothermic reaction is taking place and energy is absorbed into the system from the surroundings.
7.3: Exothermic and Endothermic Reactions - Chemistry LibreTexts Endothermic and exothermic reactions can be visually represented by energy-level diagrams like the ones in Figure 7.3. 2. In endothermic reactions, the reactants have higher bond energy (stronger bonds) than the products. Strong bonds have lower potential energy than weak bonds. Hence, the energy of the reactants is lower than that of the products.
Energy Diagrams: Describing Chemical Reactions Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DGrxn, as well as DG1* and DG2* for the first and second activation energies, respectively. Label the positions corresponding to the transition states with an asterisk.
What are Endothermic Reactions? (with Examples & Video) Energy Level Diagram of an Endothermic Reaction. The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is generally lower than that of the reactant.
Topic 5.1 Exothermic and Endothermic Reactions Heat and ... Draw an energy level diagram for a reaction in which the total energy of the reactants is 50 kJ mol-1, ... Draw a labeled enthalpy level diagram for an exothermic and endothermic reaction showing the activation energy, Ea and enthalpy change. [4] 9. (M05/S/2) In a neutralization reaction 50 cm 3 of a 0.50 moldm-3 solution of sodium hydroxide is mixed rapidly in a glass …
Energy level diagrams - Why are there energy changes in ... Energy level diagrams are used to model energy changes during reactions. They show the relative energy levels of the products and reactants. Exothermic reaction The energy level decreases in an...
Potential Energy Diagrams - Chemistry - Catalyst, Endothermic ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
Creative Diagram Of Exothermic Reaction - Glaucoma Template An energy level diagram shows whether a reaction is exothermic or endothermic. Hesss law and reaction enthalpy change. In endothermic reactions the energy of the reactants is lower than that of the products. Draw and explain energy level diagrams to represent exothermic and endothermic. Diagram of endothermic and exothermic reactions.
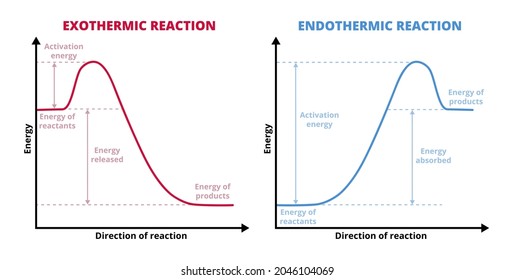
The Cold Pack: A Chilly Example of an Endothermic Reaction ... 01/06/2020 · Energy diagrams show the energy levels of reactants and products in a reaction. General energy diagrams for exothermic and endothermic reactions (©2020 Let’s Talk Science). You can see from the diagram above that the energy level of the products of an exothermic reaction is lower than the energy level of the reactants.
Endothermic and Exothermic reactions Flashcards | Quizlet Energy level diagram for an endothermic reaction. freezing water into ice cubes. exothermic - HEAT LEAVES THE WATER TO FORM ICE. boiling water . endothermic - HEAT ENTERS THE WATER TO MAKE IT BOIL. evaporation. endothermic - HEAT ENTERS THE WATER TO TURN IT INTO STEAM. baking a cake. endothermic - HEAT ENTERS, CAKE MUST BECOME …
Reaction profiles - Exothermic and endothermic reactions ... An energy level diagram. shows whether a reaction is exothermic. or endothermic. It shows the energy in the reactants and products , and the difference in …
Reaction profiles - Exothermic and endothermic reactions ... An energy level diagram shows whether a reaction is exothermic or endothermic. It shows the energy in the reactants and products, and the difference in energy between them. Exothermic reaction The...
Exothermic and Endothermic Processes | Introduction to ... Endothermic reaction. In an endothermic reaction, the products are higher in energy than the reactants. Therefore, the change in enthalpy is positive, and heat is absorbed from the surroundings by the reaction. Whether a reaction is endothermic or exothermic depends on the direction that it is going; some reactions are reversible, and when you revert the products back …
Reaction Coordinate Diagram Endothermic Vs Exothermic A reaction will be exothermic if the energy of the products is less than the energy of the Below is a reaction coordinate diagram for an endothermic reaction. A general Reaction Coordinate Diagram relating the energy of a system to leading to an exothermic reaction (∆H 0). Endothermic Reaction.
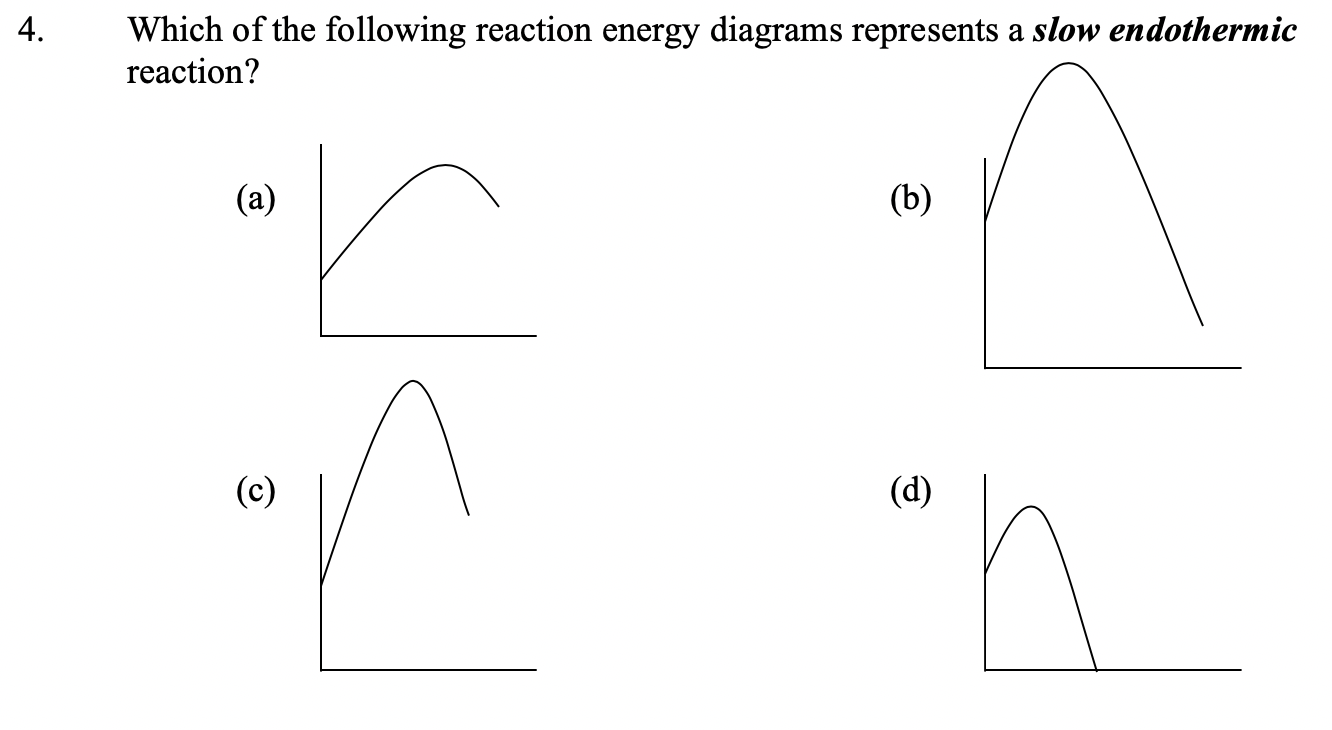
6.9: Describing a Reaction - Energy Diagrams and ... sketch the reaction energy diagram for a single-step reaction, given some indication of whether the reaction is fast or slow, exothermic or endothermic. interpret the reaction energy diagram for a single-step process (e.g., use the diagram to decide whether the reaction is exothermic or endothermic).
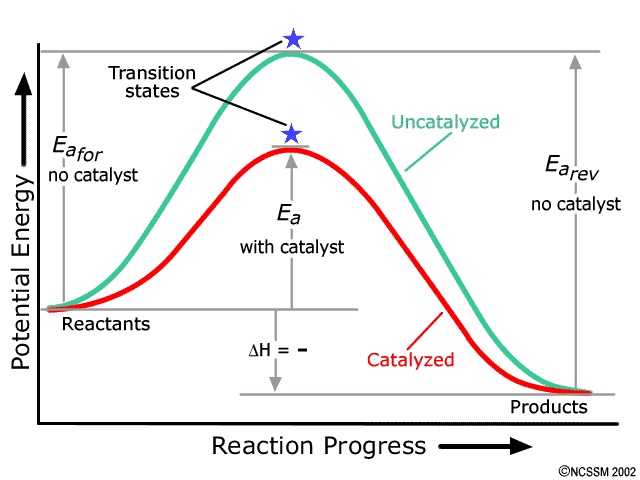
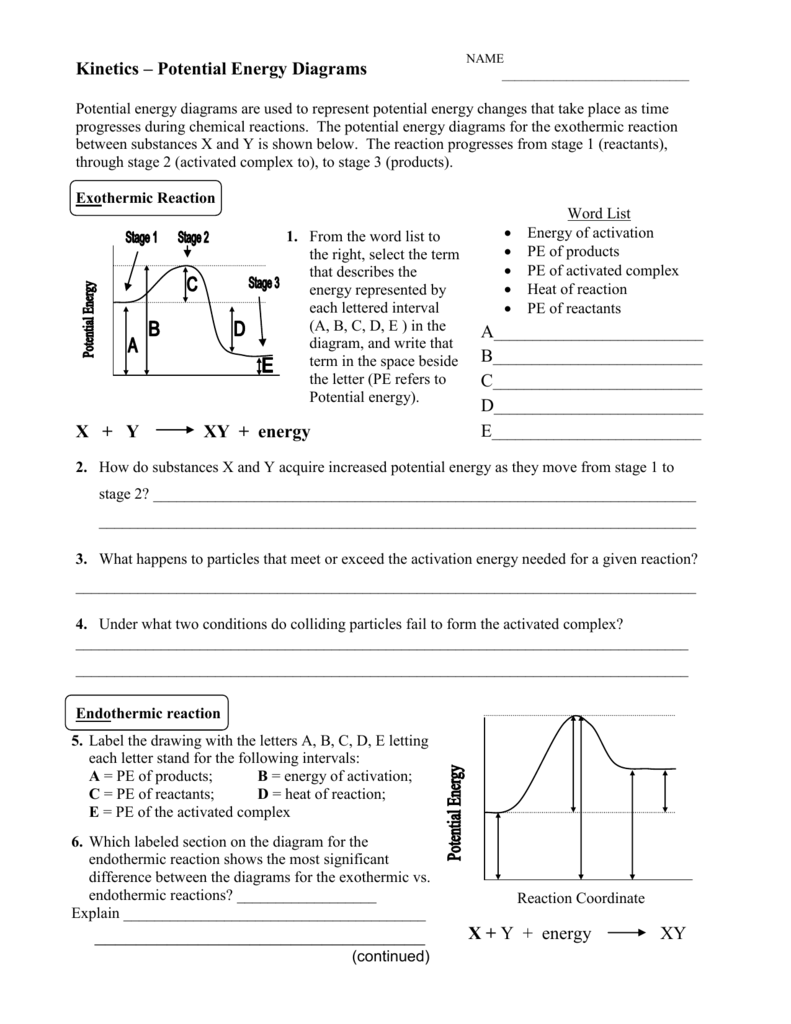
PDF Exothermic vs endothermic reaction graphs State one reason, in terms of energy, to support your answer.Answer-->Endothermic, the products have more energy than the reactants.b) On the diagram provided in your answer booklet, draw a dashed line to indicate a potential energy curve for the reaction if a catalyst is added.Answer--
Potential Energy Diagrams - Kentchemistry.com 52 Sketch the potential energy diagram for an endothermic chemical reaction that shows the activation energy and the potential energy of the reactants and the potential energy of the products. Answer--> 1/04. 16 Which statement best explains the role of a catalyst in a chemical reaction? (1) A catalyst is added as an additional reactant and is consumed but not …
Endothermic Reaction Coordinate Diagram - schematron.org Endothermic reaction. Draw an energy coordinate diagram for both an endothermic and an exothermic reaction. In Sam's case, when ammonium nitrate was dissolved in water, the system absorbed heat from the surrounding, the flask, and thus the flask felt schematron.org is an example of an endothermic reaction.
PDF 5.1 - Exothermic and Endothermic Reactions 5.1.4 - Deduce, from an enthalpy level diagram, the relative stabilities of reactants and products, and the sign of the enthalpy change for the reaction Enthalpy level diagrams, or energy profile diagrams, allow us to visualise what happens to the enthalpy of a reaction as it proceeds The total enthalpy of the reactant species is labelled H R
Exhothermic and Endothermic Reactions / Activation Energy ... • In an Exhothermic reaction, the energy constantly being released by the reaction allows the process to continue once it has been started; but, in an Endothermic reaction, the process has to suck energy in continuously from the surrounding atmosphere – and by doing so, THAT is what enables it to keep going! If you were to place the liquid reactants in an Endothermic reaction …





















0 Response to "42 Endothermic Reaction Energy Diagram"
Post a Comment