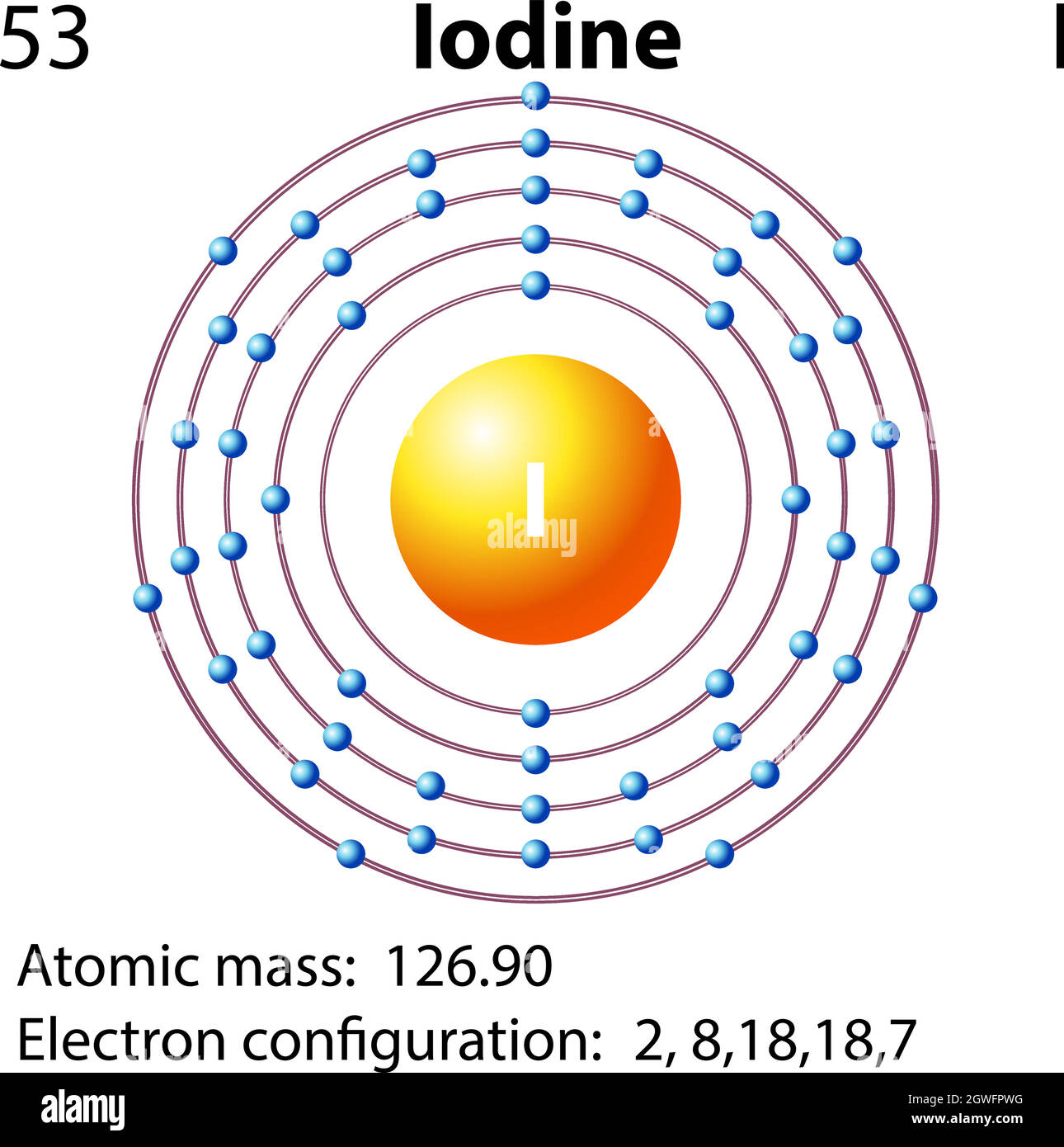
38 dot diagram for iodine
Electron Dot Diagrams and Lewis Structures Flashcards ... electron dot diagram for Phosphorus. Lewis structure for PCl₃. Lewis structure for CH₄. Lewis structure for CH₃Br. Lewis structure for F₂O. Lewis structure for IBr. 6 dots around I, single bond, 6 dots around Br. Lewis structure for NH₂Cl. N in middle, 2 dots around N, single bonded to H on both left and right, single bonded down with ... Iodine dichloride (ICl2-) lewis dot structure, molecular ... Since both iodine and chlorine atom belongs to the same periodic group (Group 17), they have same valence electrons. ∴ Total number of valence electron available for ICl2- lewis structure = 7 + 7 (2) + 1 = 22 valence electrons [∴ICl2- molecule has one iodine and two chlorine atom with one negative charge ion] 2.
Iodine Electron Dot Diagram - wiringall.com This example Total valence electrons to be "happy" = 1 iodine (8) + 3 chlorine (3 x 8). Comprehensive information for the element Iodine - I is provided by this page including scores of properties, Atomic Structure of Iodine Electron Dot Model . The left diagram shows a Lewis dot structure of sodium with. Likewise, they can be used ..
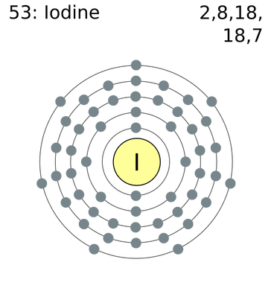
Dot diagram for iodine
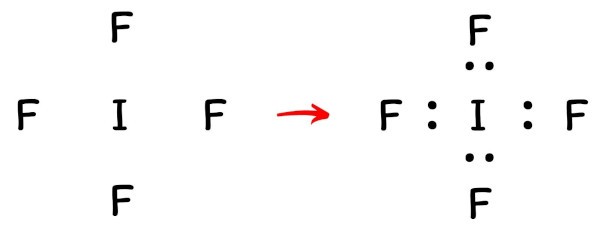
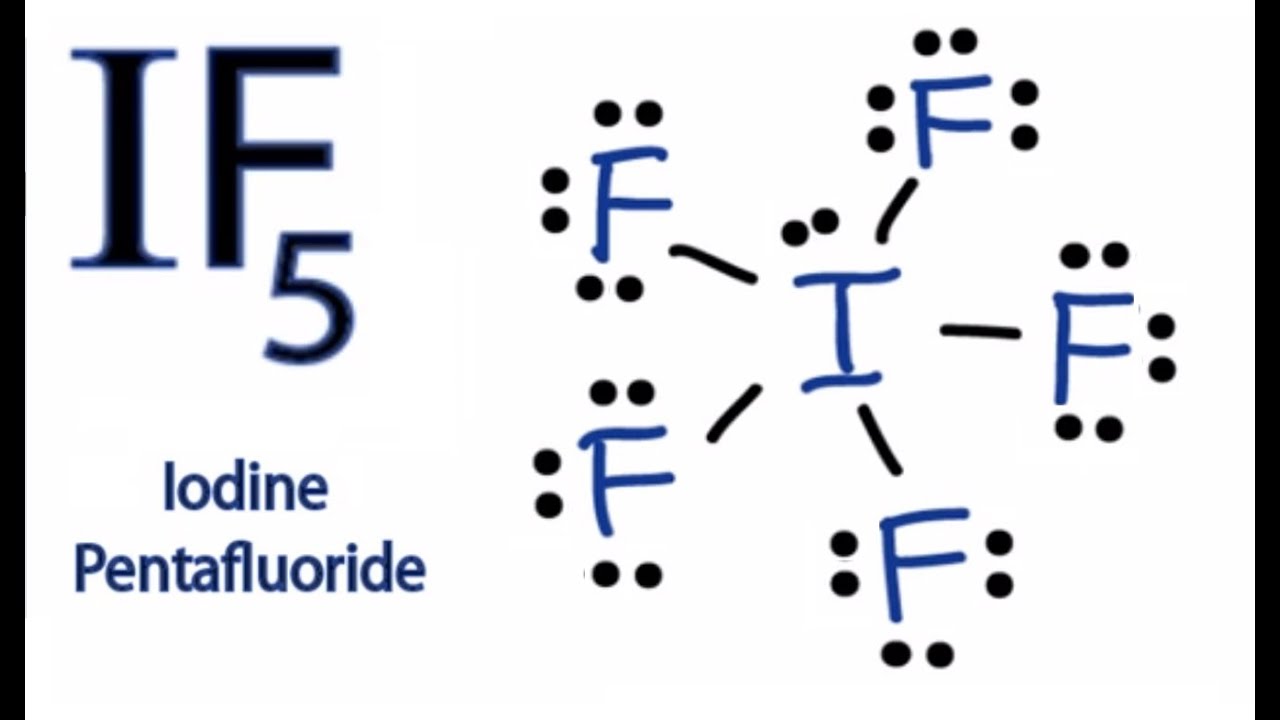
PDF Lewis Dot Structures - University of Pennsylvania School ... c) Draw the Lewis dot structure for the compound CBr 4. 2. a) Draw the Lewis dot structure for an atom of iodine. b) Using the NEED, HAVE, SHARE method, determine the number of valence electrons that would be shared between two iodine atoms. c) Draw the Lewis dot structure for the compound I 2. 3. Repeat for the compound O 2. What is the ionic bond for magnesium and iodine? When Magnesium and Iodine are combined with some water, a violet vapor is produced. The reaction of Magnesium metal and Iodine will 'yield' Magnesium Iodide. The two elements will create an ionic bond (MgI2). The two valence electrons of Mg will be distributed to each of the Iodine atoms. Lewis dot diagram for iodine? - Answers the Lewis dot diagram is a table used for the elements, and it shows you how many valence electrons there are. What is the Lewis dot diagram of IF3? The dot structure of Iodine triflouride starts ...
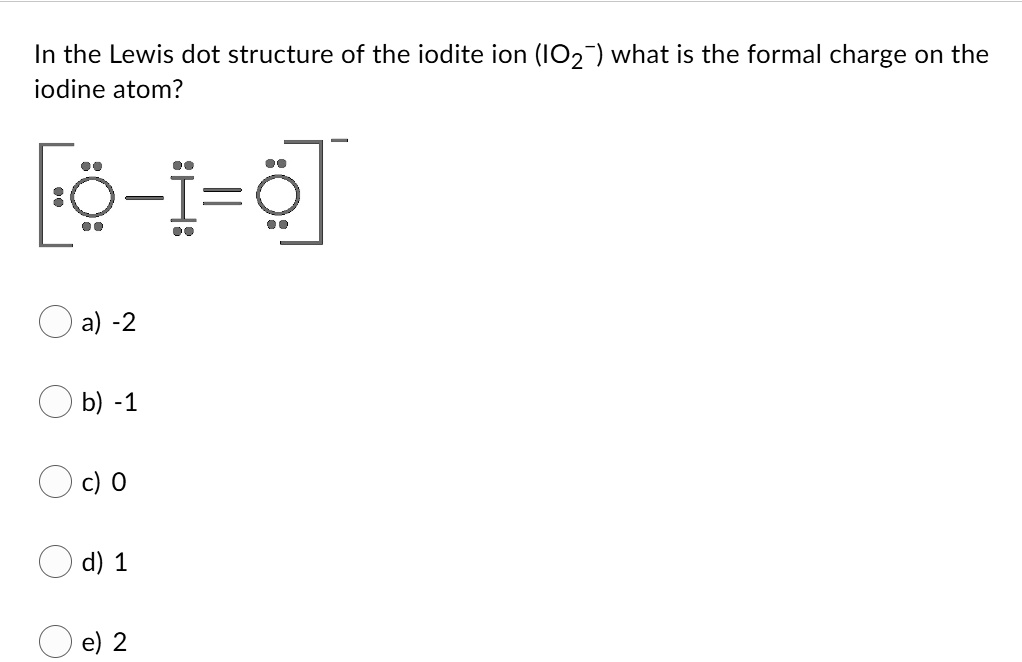
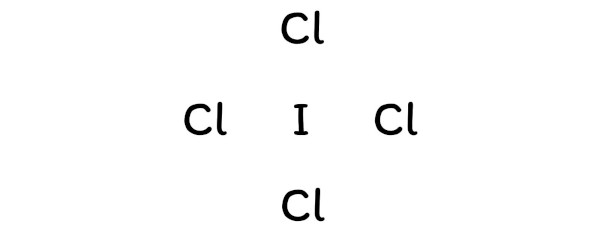
Dot diagram for iodine. Give The Lewis Dot Structure For Icl2-, Chemical Bonding ... Lewis Dot Structure For Icl2-. ICl2- lewis structure consists of one iodine atom in ~ the center position whereas 2 chlorine atoms at the surrounding position. There room three lone pairs present on the central atom of ICl2- lewis structure. Also, the iodine central atom in ICl2- lewis framework violates the octet as it is holding an ext than 8 ... What should the electron dot diagram for iodine look class ... Hint: The electron dot diagram is the diagram which clearly shows the bonding between the atoms of a molecule along with the lone pairs of electrons, if they exist in the molecule. Iodine is the molecule consisting of two atoms of iodine bonded together satisfying both the planes. Complete answer: Let us study the concept; PPTX Topic: Lewis Dot Diagrams for Ionic Compounds What is the ionic compound formed from calcium and iodine? Calcium - metal - 2 valence electrons - loses both electrons [Ca]+2. Iodine - nonmetal - 7 valence electrons - gains 1 electron ... Topic: Lewis Dot Diagrams for Ionic Compounds Last modified by: Solved 1) Draw the Lewis dot structures for the following ... 1) Draw the Lewis dot structures for the following compounds: O2, ICI (Iodine/Chlorine), CIF3 (Chlorine/Fluorine). Which compound (s) contain atoms that do not obey the octet rule? 2) Draw all possible resonance structures for the molecule, NCS (assume these are the correct connections). 3) Assign formal charges to each atom in the above ...
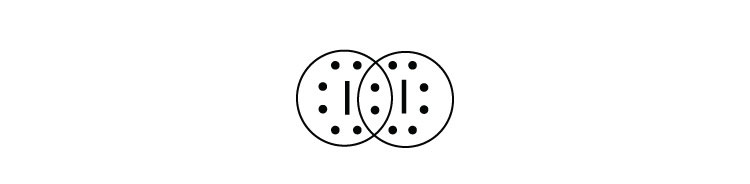
I2 Lewis Structure - How to Draw the Dot Structure for I2 ... A step-by-step explanation of how to draw the I2 Lewis Dot Structure (Iodine Gas).For the I2 structure use the periodic table to find the total number of val... Iodine Electron Dot Diagram - schematron.org iodine has 7 valence electrons. So you draw seven dots, 2 on each side of the letter I. and on one side you just put one dot, I think its best to put. The left diagram shows a Lewis dot structure of sodium with. ASK A BRAND Likewise, they can be used .. The left shows an iodine atom with one lone pair. Iodine Lewis Dot Diagram - schematron.org The left diagram shows a Lewis dot structure of sodium with. Likewise, they can be used .. The left shows an iodine atom with one lone pair. When we write the . Comprehensive information for the element Iodine - I is provided by this page including scores of properties, Atomic Structure of Iodine Electron Dot Model . Lewis Dot Diagram Iodine Lewis Dot Diagram Iodine Comprehensive information for the element Iodine - I is provided by this page including scores of properties, Atomic Structure of Iodine Electron Dot Model . The left diagram shows a Lewis dot structure of sodium with. Likewise, they can be used .. The left shows an iodine atom with one lone pair. When we write the .
How to Draw the Lewis Dot Structure for I: Iodine - YouTube A step-by-step explanation of how to draw the I Lewis Dot Structure.For the I structure use the periodic table to find the total number of valence electrons ... What should the electron dot diagram for iodine look like ... What should the electron dot diagram for iodine look like? Chemistry Covalent Bonds Drawing Lewis Structures. 1 Answer Jahan Psyche Oct 24, 2015 Answer link. Related questions. What are lewis dot structures used for? What is the lewis structure for #SO_2#? How do you draw the lewis structure for ions? ... Iodine monochloride | ICl - PubChem Iodine monochloride solution, 1 M in acetic acid. Iodine monochloride, 99.998% trace metals basis. Q414607. Iodine monochloride solution, 1.0 M in methylene chloride. Iodine monochloride, ACS reagent, 1.10+/-0.1 I/Cl ratio basis. Iodine monochloride, approx. 0.22N soln. in glacial acetic acid. Solved 1. Draw a Lewis dot diagram for an iodine molecule ... Draw a Lewis dot diagram for an iodine molecule. 2. Draw a Lewis dot diagram for a hydrogen molecule. 3. True or false: When drawing a covalent bond, it is proper to use a line connect atoms and a line represents 2 electrons. 4. In your own words, describe the difference between a bond that is nonpolar covalent and polar covalent. Cite one ...
› category › celebritiesCelebrities Archives - Hollywood.com Click to get the latest Celebrities content. Sign up for your weekly dose of feel-good entertainment and movie content!
What is the Lewis dot structure for HOI.? 2 Answers. O goes in the middle. First, that's the order they are written in. Second, oxygen forms 2 bonds, so it can form a bond with H and with I. Starting with that, finishing the Lewis structure is very easy. Just account for 1 + 6 + 7 = 14 valence electrons in the drawing. Kleiner.
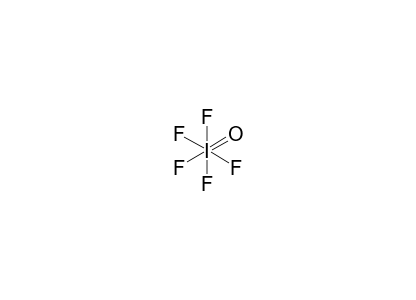
PDF 1)Draw an electron-dot diagram for each of the following ... At STP, iodine, I2, is a crystal, and fluorine, F2, is a gas. Iodine is soluble in ethanol, forming a tincture of iodine. A typical tincture of iodine is 2% iodine by mass. Draw a Lewis electron-dot diagram for a molecule of I2. 23)Draw a Lewis electron-dot diagram for a molecule of phosphorus trichloride, PCl3
PDF Lewis electron dot diagram for potassium iodide Chemical elements.com - iodine (I) How draws a Lewis Dot diagram for the Iodide Ionic Bonding of Potassium - DocSTOC Documents, Models, Shapes, Ebook. Chapter 3 . We put together the two ions to complete the Lewis structure for Ki. We will then design Lewis for the CL ION and add brackets. Choose Lewis Electron Lewis's DOT diagram for an atom ...
Boron triiodide (BI3) lewis dot structure, molecular ... The lone pair are represented as dots in the lewis diagram. According to the BI3 lewis structure, there is a total of 18 dots present (6 dots on each iodine atom). Hence, the number of lone pairs in the BI3 lewis structure is 9. The total number of bond pairs present in the lewis structure of BI3?
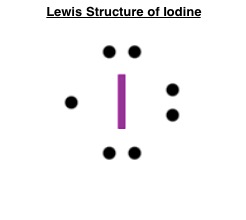
what is the lewis dot structure for iodine? what is the lewis dot structure for iodine? Iodine is in group 7, so there will be 7 electrons 2 on top, 2 to the left, 2 on the bottom and 1 to the right. Iodine has seven valence electrons, because it is in group seven on the periodic table and is a halogen.
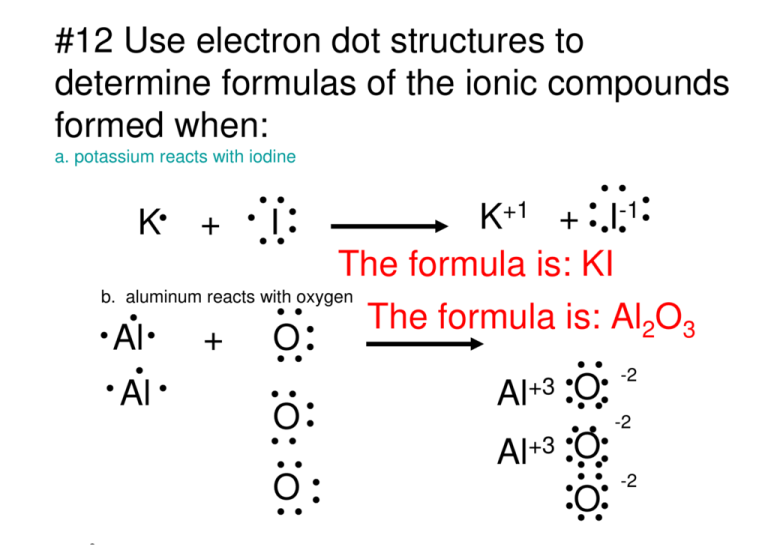
How to draw KI Lewis Structure? - Science Education and ... To sketch the KI Lewis structure by following these instructions: Step-1: KI Lewis dot Structure by counting valence electrons on the iodine atom. Step-2: Lewis Structure of KI for counting valence electrons around the terminal potassium atoms. Step-3: Lewis dot Structure for KI generated from step-1 and step-2.
Draw a Lewis dot diagram for an iodine molecule. Draw a ... Draw a Lewis dot diagram for an iodine molecule. Draw a Lewis dot diagram for a hydrogen molecule. True or false: When drawing a covalent bond, it is proper to use a line connect atoms and a line represents 2 electrons. In your own words, describe the difference between a bond that is nonpolar covalent and polar covalent.
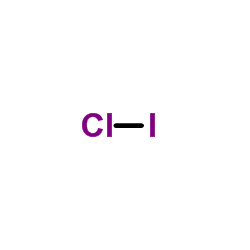
ICl Lewis Structure - How to draw the Electron Dot ... For Iodine we have 7 valence electrons, and 7 for the Chlorine; total of 14 valence electrons for the ICl Lewis structure. We'll put the Iodine here, and the Chlorine right next to it. We have a total of 14 valence electrons. We'll put 2 between atoms to form the chemical bond, and we'll go around the outside.
Iodine | I2 - PubChem Create. 2004-09-16. Iodine is a naturally occurring element found in sea water and in certain rocks and sediments. There are non radioactive and radioactive forms of iodine. Iodine is used as a disinfectant for cleaning surfaces and storage containers and is used in skin soaps and bandages, and for purifying water.
what is the lewis dot structure for iodine? - Soetrust Iodine has seven valence electrons, because it is in group seven on the periodic table and is a halogen. The dot structure would be the element symbol, I, surrounded by seven dots, three on two sides and one on the remaining side. It doesn't matter which side has the single electron (dot). Definition of Burial - What it is, Meaning and Concept.
Lewis dot diagram for iodine? - Answers the Lewis dot diagram is a table used for the elements, and it shows you how many valence electrons there are. What is the Lewis dot diagram of IF3? The dot structure of Iodine triflouride starts ...
What is the ionic bond for magnesium and iodine? When Magnesium and Iodine are combined with some water, a violet vapor is produced. The reaction of Magnesium metal and Iodine will 'yield' Magnesium Iodide. The two elements will create an ionic bond (MgI2). The two valence electrons of Mg will be distributed to each of the Iodine atoms.
PDF Lewis Dot Structures - University of Pennsylvania School ... c) Draw the Lewis dot structure for the compound CBr 4. 2. a) Draw the Lewis dot structure for an atom of iodine. b) Using the NEED, HAVE, SHARE method, determine the number of valence electrons that would be shared between two iodine atoms. c) Draw the Lewis dot structure for the compound I 2. 3. Repeat for the compound O 2.



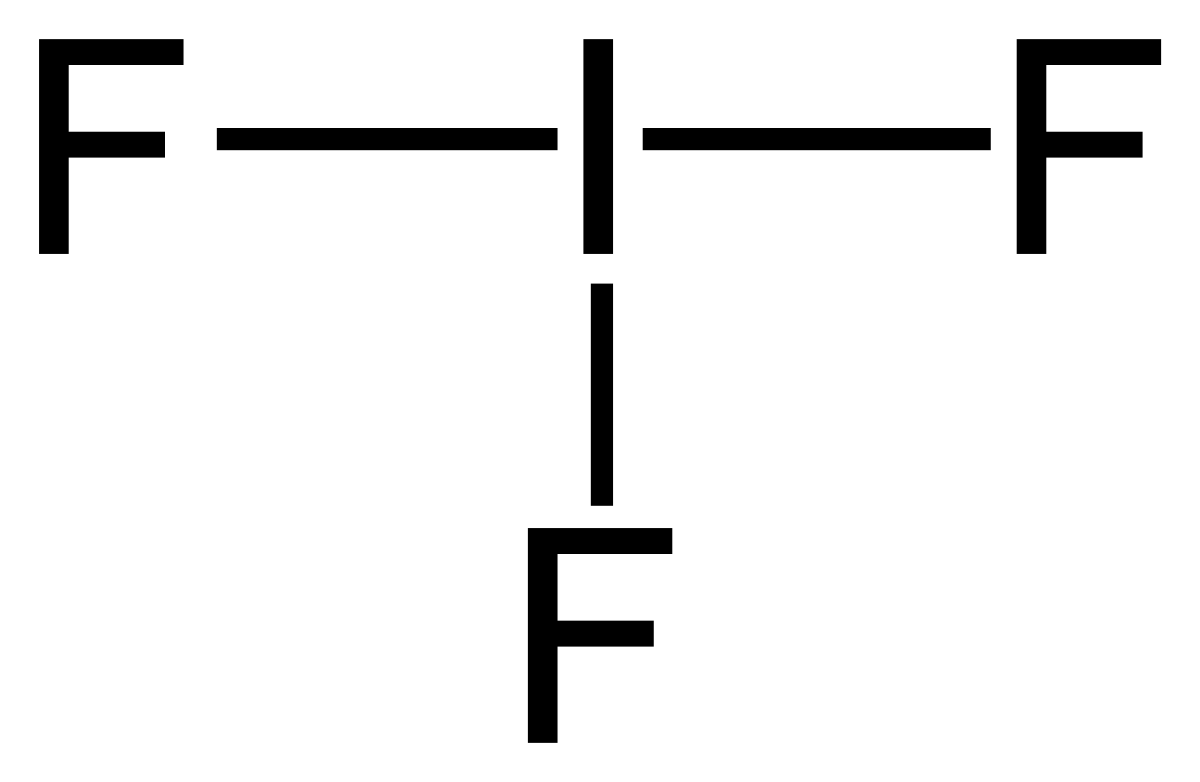
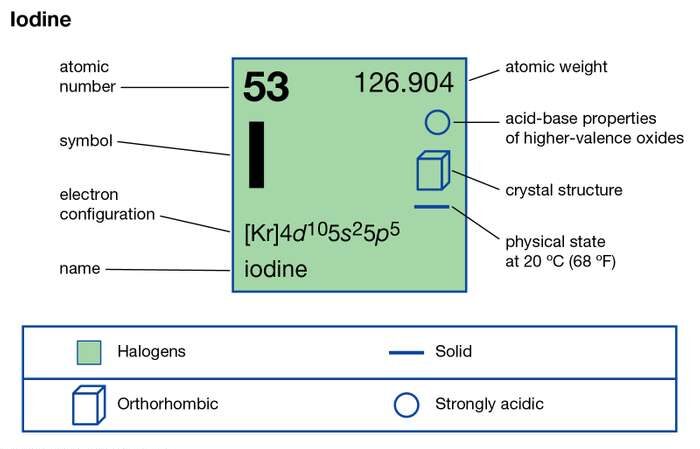

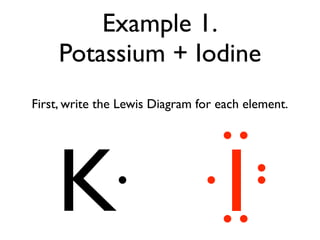
:max_bytes(150000):strip_icc()/ICl3_LD-56a12a2b3df78cf77268034c.png)

















0 Response to "38 dot diagram for iodine"
Post a Comment