38 co3+ orbital diagram
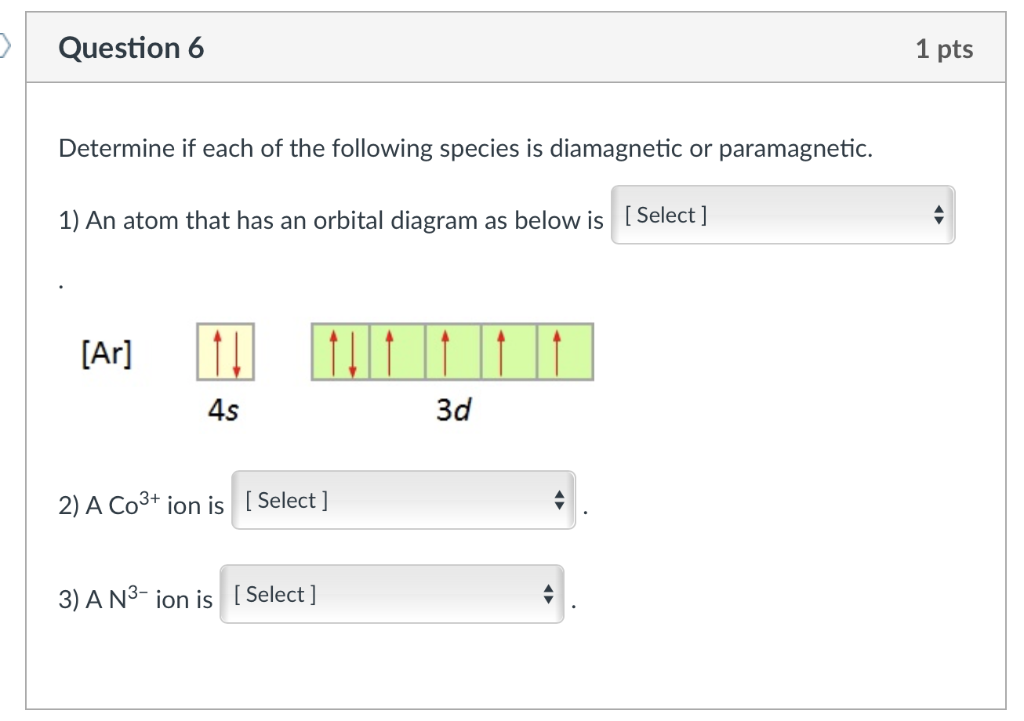
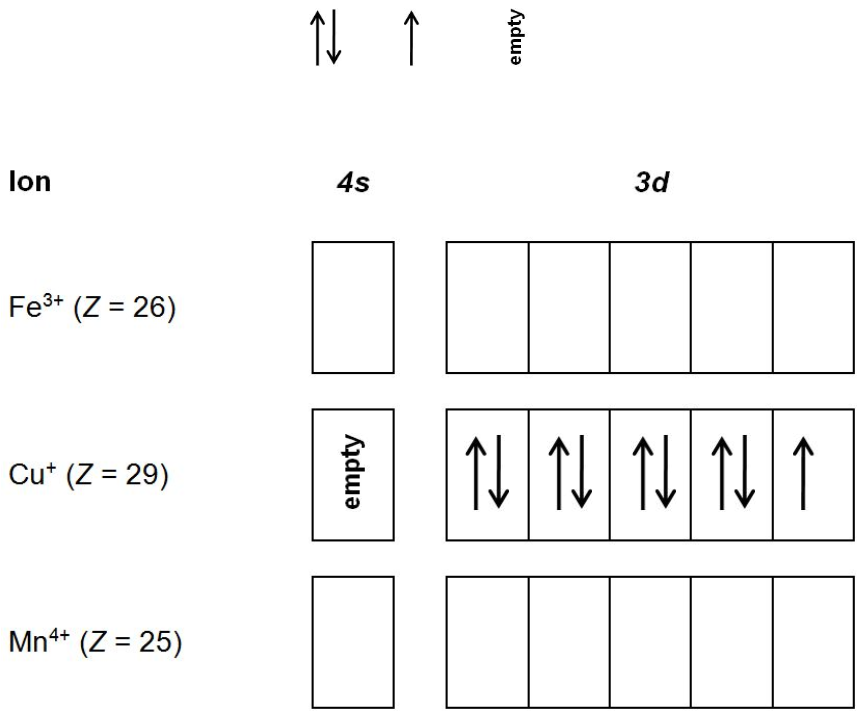
What is the electron configuration of "Co"^"3+"? | Socratic The electron configuration of Co3+ is [Ar]4s3d5. Co is in Period 4 of the Periodic Table, and Ar is the preceding noble gas. Cobalt is also in Group 9, so it must have 9 valence electrons. The valence shell configuration is therefore 4s23d7, and the core notation is. When a transition metal forms an ion, the s electrons are removed before the d ... SOLVED:Complete the partial orbital diagrams for ions ... Complete the partial orbital diagrams for ions: Complete the partial orbital diagrams for the ions indicated. Fill orbitals from left to right and do not leave any boxes unfilled: Ion 4s 3d Ap Co3+ (Z = 27) Cu2+ (Z = 29) Sez- (Z = 34)
Electronic Structure of Atoms (Electron Configurations ... The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2 s and then 2 p , 3 s , and 3 p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms.

Co3+ orbital diagram
How bond order is calculated and why co+ has bond ... - BYJUS Bond order=1/2 (bonding−anti-bonding) According to molecular orbital diagram, the bond order of CO+ is 3.5. The highest occupied molecular orbital is sigma*2s MO. In the case oc CO, the 2s atomic orbital on oxygen is much lower than the energy than the 2s atomic orbital of carbon. This discrepancy of energy allows the pi2px & pi2py BMO to ... Carbon Orbital diagram, Electron configuration, and ... Orbital diagram:-A orbital diagram is simply a pictorial representation of the arrangement of electrons in the orbital of an atom, it shows the electrons in the form of arrows, also, indicates the spin of electrons.Electron configuration:- Electron configuration is the arrangement of electrons in atomic orbitals.It shows the electrons in numbers, It doesn't show the details on the spin of ... Co2+ Orbital Diagram A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. The valence electron configuration of "O" is ["He"] 2s^2 2p^4.
Co3+ orbital diagram. Cobalt(Co) electron configuration and orbital diagram The 3p orbital is now full. So, the next two electrons will enter the 4s orbital and the remaining seven electrons will enter the 3d orbital. Therefore, the cobalt(Co) electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 3d 7 4s 2. How to write the orbital diagram for cobalt(Co)? Electron Configuration for Co, Co2+, and Co3+ (Cobalt and ... To write the configuration for the Cobalt ions, first we need to write the electron configuration for just Cobalt (Co). We first need to find the number of ... What is the hybridization of carbon in co3 2 CO3 2- is carbonate. a carbonate is a salt of carbonic acid (H2CO3),characterized by the presence of the carbonate ion, a polyatomic ion with the formula of CO3 2-.CO32- is an anion (a negative ion) seen frequently in chemistry.In the CO32- Lewis structure carbon is the least electronnegative element. How To Draw Molecular Orbital Diagram Of Co - Drawing ... A) draw a molecular orbital (mo) diagram for co and show the filling of electrons. Let's take [co (nh3)6]3+ as an example. For the homonuclear diatomic #o_2#, we simply have two copies of this atomic orbital diagram far apart at first. Electronic configuration of co molecule is: Draw the orbital diagram for the ion co2+.
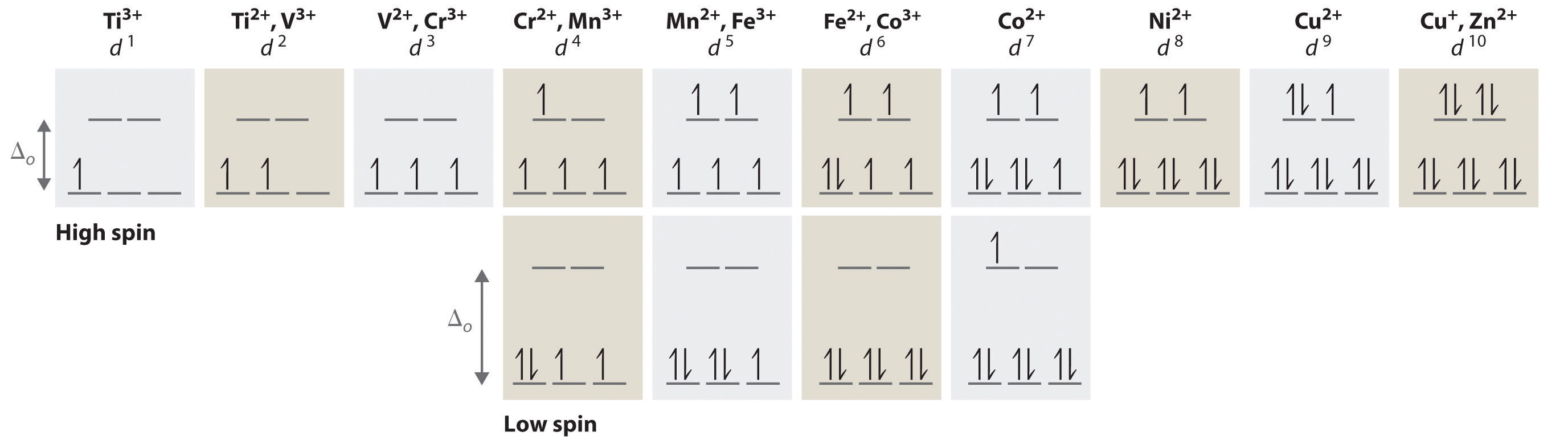
What is the orbital notation for cobalt? - FindAnyAnswer.com Orbital Diagram 1s ↿⇂ 2s ↿⇂ 2p ↿⇂ ↿⇂ ↿⇂ 3s ↿⇂ 3p ↿⇂ ↿⇂ ↿⇂ 3d ↿⇂ ↿⇂ ↿⇂ ↿⇂ ↿⇂ 4s ↿⇂ 4p ↿⇂ ↿ ↿ 4d 4f: ... The electron configuration of Co3+ is [Ar]4s3d5 . Co is in Period 4 of the Periodic Table, ... Carbonate | CO3-2 - PubChem Carbonate | CO3-2 | CID 19660 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety ... Mo3+ Orbital Diagram - schematron.org Mo3+ Orbital Diagram. That's why Mo3+ has 39 electrons instead of 42 in its ground state electron The normal electron configuration of zinc is [Ar] 3d 10 4s 2, with 2 4s orbital. However, even though the 5s orbital is lower in energy than the 4d orbital, the electrons in the 4d orbitals shield the electron in the 5s orbitals. PDF Coordination Chemistry II: Ligand Field Theory Continued electrons into the same orbital •Πeis a stabilizing energy for electron exchange associated with two degenerate electrons having parallel spin total 3 e 0 c eg* t2g d4HS eg* t2g d8 eg* t2g d6LS total 7 e 3 c total 6 e 3 c LFSE 3 0.4 O 10.6 O 0.6 O LFSE 6 0.4 O 20.6 O
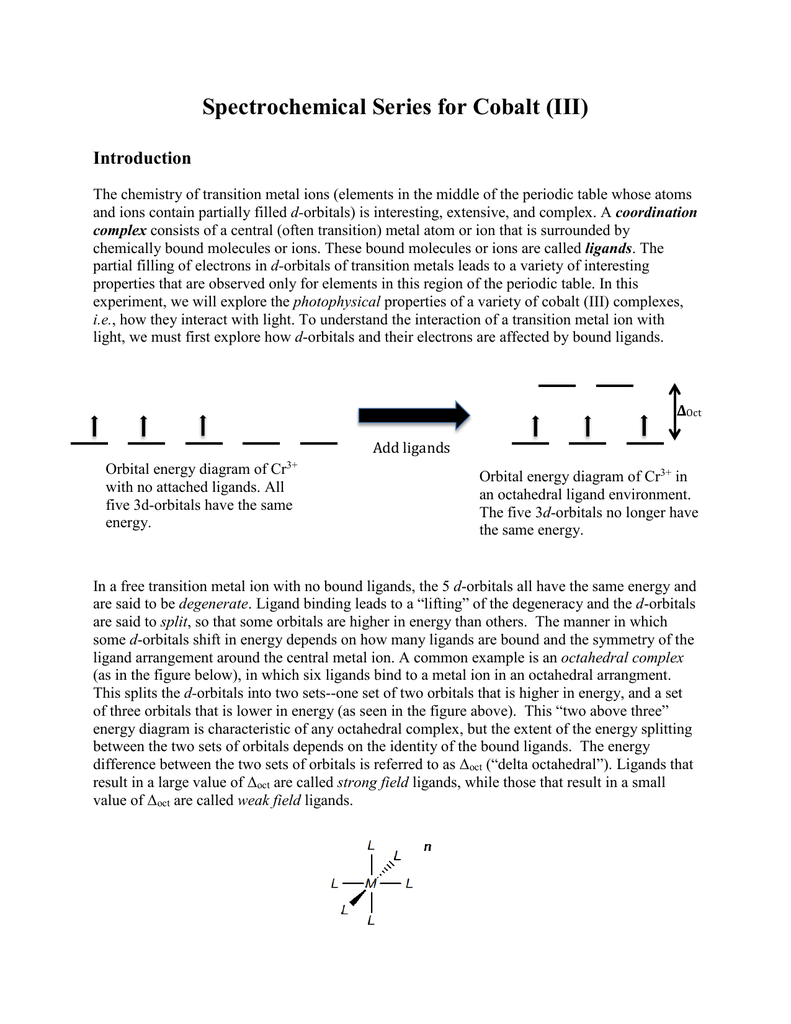
co3 2 molecular orbital diagram - danklass.com January 10, 2021 CO32- Lewis Structure, Molecular Geometry, Hybridization ... CO32- Molecular Orbital (MO) Diagram What is MO theory? Molecular Orbital Theory is a concept of quantum mechanics that is used to decipher the chemical bonding nature inside different molecular structures. This is a complex yet useful tool that helps in sketching MO diagrams for better understanding. PDF Spectrochemical Series for Cobalt (III) - Texas A&M University 3. 3+Using your answer from Question 2, draw a d-orbital splitting diagram for low-spin Co similar to the one given for Cr3+ 3+in the introduction to this experiment. Almost all Co complexes are low-spin, with a minimum number of unpaired electrons. (Refer to your text for further explanation.) 4. Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ...
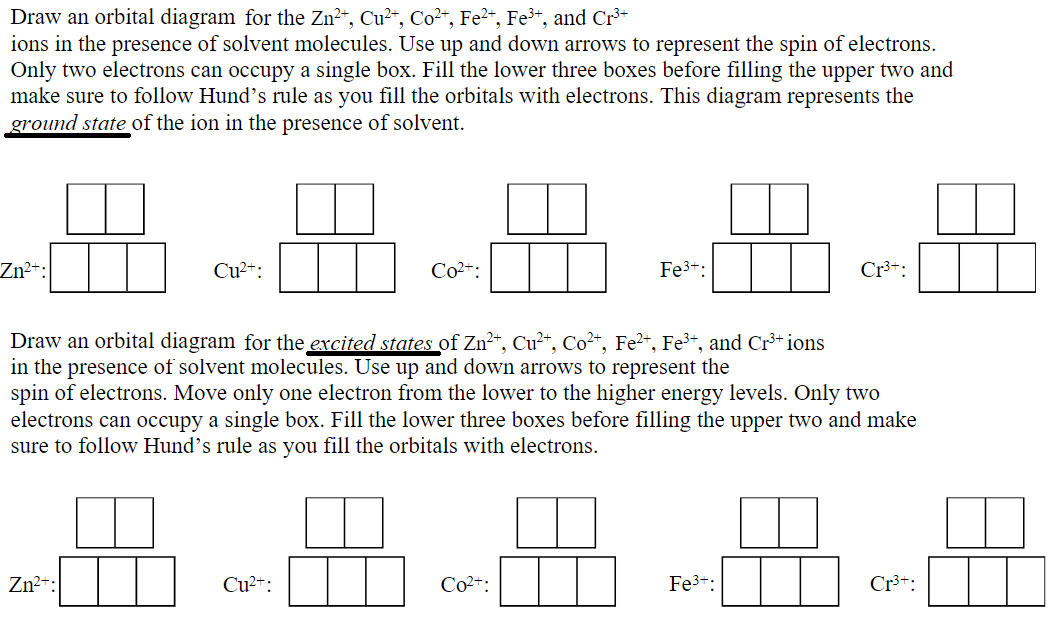
Solved Draw an orbital diagram for the Zn2+, Cu2+, Co2 ... Transcribed image text: Draw an orbital diagram for the Zn2+, Cu2+, Co2+, Fe2+, Fe3+, and Cr3+ ions in the presence of solvent molecules. Use up and down arrows to represent the spin of electrons. Only two electrons can occupy a single box. Fill the lower three boxes before filling the upper two and make sure to follow Hund's rule as you fill the orbitals with electrons.
Solved What is the molecular orbital diagram for a ... This problem has been solved! See the answer. See the answer See the answer done loading. What is the molecular orbital diagram for a carbonate ion? CO3 2- Please include labels for the orbitals like T2g, pi, pi anti-bonding etc. Expert Answer.
How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
PDF Molecular Orbital Approach to Bonding MOLECULAR ORBITAL APPROACH Basis of VB approach: overlap orbitals in each bond separately. Each bond is LOCALISED between two atoms. In molecular orbital (MO) approach - overlap orbitals for the whole molecule - bonding is therefore DELOCALISED. We will look first at DIATOMIC MOLECULES and only later move on to POLYATOMIC MOLECULES.
PDF Exercise 4.4 Pi Molecular Orbital Energy Level Diagrams ... 1. (a) Construct a pi molecular orbital energy level diagram for the allyl cation (𝐶𝐶. 3. 𝐻𝐻. 5+). Label the HOMO and LUMO. Hint: You drew pictures of all the π MOs in question 2 of Exercise 4.3. (b) Based on your pi molecular orbital energy level diagram and on the pictures of the π MOs
Draw The Orbital Diagram For The Ion Co2+ Part B. Draw the orbital diagram for the ion Co2+. Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at. Ni2+ Draw the d-orbital splitting diagrams for the octahedral complex ions of each of the following. a. Zn2+ b. Co2+ (high and low spin) c. Ti3+ the FT ligand.
Answered: For the carbonate ion, CO3 2− 1- Draw… | bartleby For the carbonate ion, CO3 2−. 1- Draw the electron orbital diagram for the valence electrons of the central carbon. before and after hybridization. 2- Identify which carbon and oxygen electron orbitals overlap to create each single and double C-O bond in the structure. Expert Solution.
What is molecular orbital diagram of CO? - handlebar ... What is molecular orbital diagram of CO? Carbon monoxide MO diagram. Carbon monoxide is an example of a heteronuclear diatomic molecule where both atoms are second-row elements. The valence molecular orbitals in both atoms are the 2s and 2p orbitals. The molecular orbital diagram for carbon monoxide (Figure 5.3.
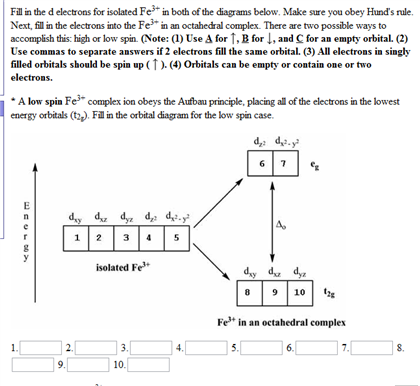
What is the electron configuration of a cobalt 3+ ion? Is ... Answer (1 of 12): Transition metals, when losing electrons, first lose s electrons and then d electrons. Here are electron configurations of Co, Co+, Co2+ and Co3+: Co [Ar] 3d7 4s2 Co+ [Ar] 3d7 4s1 Co2+ [Ar] 3d7 Co3+ [Ar] 3d6 <--- answer to your question
PDF Hybrid Molecular Orbitals - University of Illinois Urbana ... molecular orbital diagram as a non-bonding molecular orbital. 7. There are a total of 6 electrons to add to the molecular orbital diagram, 3 from boron and 1 from each hydrogen atom. sp Hybrid Orbitals in BeH2 1. The Lewis structure shows that the beryllium in BeH 2 makes 2 bonds and has no lone pairs. It is a
Co2+ Orbital Diagram A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. The valence electron configuration of "O" is ["He"] 2s^2 2p^4.
Carbon Orbital diagram, Electron configuration, and ... Orbital diagram:-A orbital diagram is simply a pictorial representation of the arrangement of electrons in the orbital of an atom, it shows the electrons in the form of arrows, also, indicates the spin of electrons.Electron configuration:- Electron configuration is the arrangement of electrons in atomic orbitals.It shows the electrons in numbers, It doesn't show the details on the spin of ...
How bond order is calculated and why co+ has bond ... - BYJUS Bond order=1/2 (bonding−anti-bonding) According to molecular orbital diagram, the bond order of CO+ is 3.5. The highest occupied molecular orbital is sigma*2s MO. In the case oc CO, the 2s atomic orbital on oxygen is much lower than the energy than the 2s atomic orbital of carbon. This discrepancy of energy allows the pi2px & pi2py BMO to ...


















0 Response to "38 co3+ orbital diagram"
Post a Comment