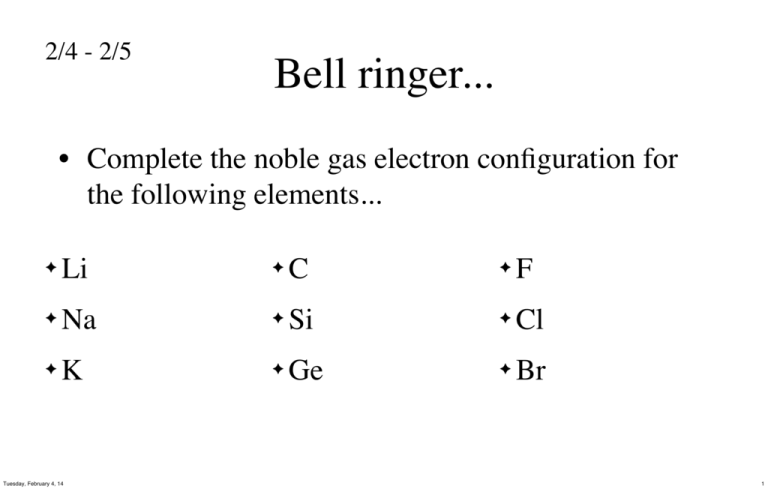
42 Arsenic Electron Dot Diagram
Electron Dot Diagram Arsenic - Wiring Diagram Pictures The answer would be Arsenic (As). An electron-dot diagram is a graphical representation of the valence electrons of a certain element. The chemical symbol of an element placed in the middle and the valence electrons are represented by dots/5 (7). AsF3 Lewis Structure, Molecular Geometry, Hybridization ... Arsenic is in group 5 of the periodic table with the electronic configuration [Ar] 3d¹⁰4s²4p³. Therefore, the single Arsenic atom contributes 5 x 1 = 5 valence electrons. Fluorine is a halogenic compound. It belongs to group 17 of the periodic table and has the electronic configuration [He] 2s22p5.
AsF5 lewis structure, Molecular geometry, Polar or ... AsF5 lewis structure is made up of one Arsenic atom situated in a central position and five fluorine atoms that spaced evenly around the central atom. There is a total of 10 bonding electrons and 30 nonbonding electrons present in the lewis structure of AsF5.

Arsenic electron dot diagram
How to draw AsCl3 Lewis Structure? - Science Education and ... In the AsCl3 Lewis structure diagram, we always begin by introducing valence electrons from the central Arsenic atom (in step1). As a result, wrap around the central Arsenic atom's bond pair valence electrons first (see figure for step1). The Arsenic atom in the molecule gets only 8 electrons around its molecular structure. AsF3 Lewis Structure - How to Draw the Dot Structure for ... The Lewis structure for AsF 3 is similar to AsCl 3 structure. Since they are in the same Group on the periodic table they each have the same number of electrons their structures are similar. The Arsenic atom goes in the center of the Lewis structure since it is the least electronegative atom. PLEASE HURRY WILL MARK BRAINLIEST !!!!!!!!!!!The electron ... The answer would be Arsenic (As). An electron-dot diagram is a graphical representation of the valence electrons of a certain element. The chemical symbol of an element placed in the middle and the valence electrons are represented by dots. The number of dots is equal to the number of valence electrons.
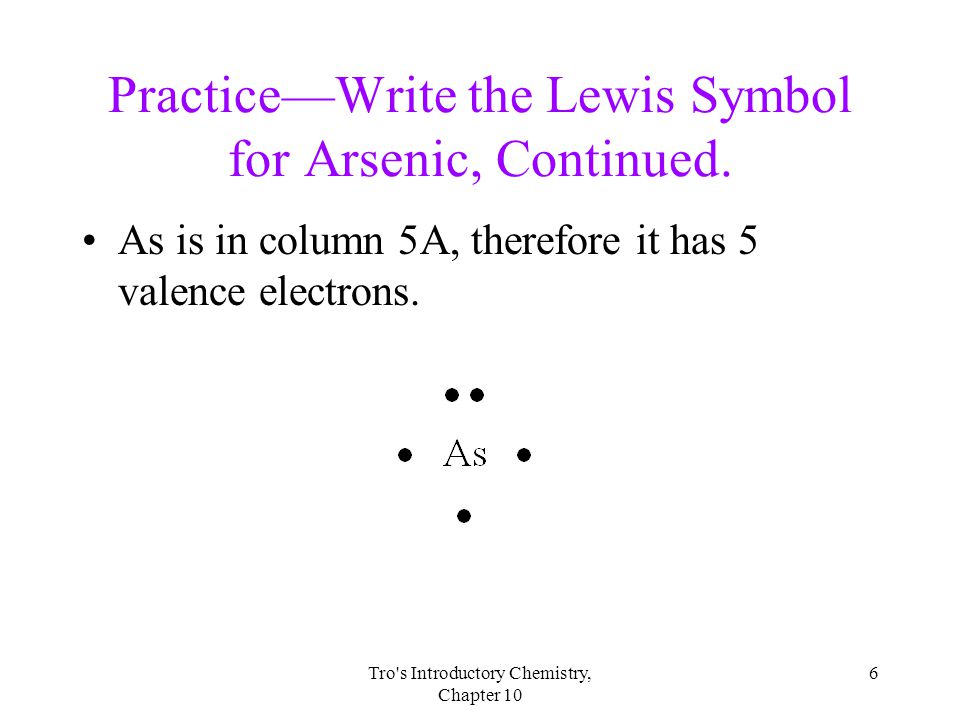
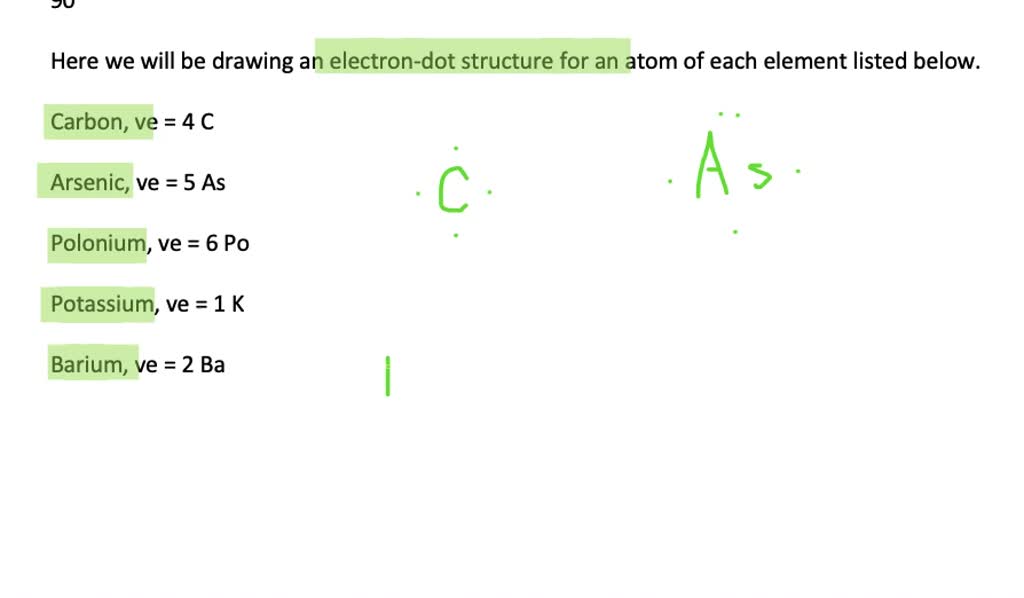
Arsenic electron dot diagram. What is Lewis dot structure for arsenic ... There should be 5 dots on the electron dot structure of arsenic- Arsenic is in column VA in the periodic table so it will have 5 valence electrons. What is the the shape molecular geometry of pbr4 +? Question: The Lewis diagram for PBr_4^+ is: The electron-pair geometry around the P atom in PBr_4^+ is There are lone pair(s) around the central ... Which elements have fewer than four dots in their electron ... Explanation: Electron dot diagrams are the diagrams which represent the valence electrons in an element. The electrons are represented by the dots in these diagrams. Valence electrons in Arsenic (As) = 5 Valence electrons Strontium (Sr) = 2 Valence electrons in Krypton (Kr) = 8 Valence electrons in Gallium (Ga) = 3 Valence electrons in Tin (Sn) = 4 ARSENIC TRIIODIDE Structure - AsI3 - Over 100 million ... The 2D chemical structure image of ARSENIC TRIIODIDE is also called skeletal formula, which is the standard notation for organic molecules. The carbon atoms in the chemical structure of ARSENIC TRIIODIDE are implied to be located at the corner(s) and hydrogen atoms attached to carbon atoms are not indicated - each carbon atom is considered to be associated with enough hydrogen atoms to ... AsH3 Lewis Structure - How to Draw the Dot Structure for ... The Lewis structure for AsH 3 is similar to AsF 3 structure. The Arsenic atom goes in the center of the Lewis structure since it is the least electronegative atom. Remember that Hydrogen (H) atoms always go on the outside of a Lewis structure. For the AsH 3 Lewis structure there are a total of 8 valence electrons available.
Arsenic pentoxide | As2O5 - PubChem Arsenic pentoxide | As2O5 | CID 14771 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities ... Periodic Table of Elements: Arsenic - EnvironmentalChemistry ... Atomic Structure of Arsenic ; Atomic Radius: 1.33Å ; Atomic Volume: 13.1cm3/mol ; Covalent Radius: 1.2Å ; Cross Section (Thermal Neutron Capture)σa/barns: 4.3 ... Arsenic trifluoride - AsF3 Lewis Structure Lewis structure of Arsenic trifluoride (AsF 3) contains three As-F bonds. Arsenic atom is located as the center atom in the molecule and each fluorine atom is located around arsenic atom. Each Fluorine atom has 3 lone pairs and also Arsenic atom has one lone pair. We will learn how to draw the lewis structure of AsF3 step by step in this tutorial. Electron Dot Diagram: study guides and answers on Quizlet Electron Dot Diagram. Quizlet is the easiest way to study, practice and master what you're learning. Create your own flashcards or choose from millions created by other students. More than 50 million students study for free with the Quizlet app each month.
How many dots should be indicated in the electron dot ... There should be 5 dots on the electron dot structure of arsenic- Arsenic is in column VA in the periodic table so it will have 5 valence electrons. T... How many Lewis dots does arsenic have ... The Arsenic atom goes in the center of the Lewis structure since it is the least electronegative atom. Remember that Hydrogen (H) atoms always go on the outside of a Lewis structure. For the AsH3 Lewis structure there are a total of 8 valence electrons available. How to Draw the Lewis Dot Structure for AsBr3: Arsenic ... A step-by-step explanation of how to draw the AsBr3 Lewis Dot Structure.For the AsBr3 structure use the periodic table to find the total number of valence el... Arsenic Lewis Structure - ash3 lewis structure how to draw ... Arsenic Lewis Structure. Here are a number of highest rated Arsenic Lewis Structure pictures on internet. We identified it from obedient source. Its submitted by handing out in the best field. We put up with this kind of Arsenic Lewis Structure graphic could possibly be the most trending subject later than we share it in google gain or facebook.
Electron Dot Diagram Arsenic - Wiring Diagrams Electron Dot Diagram Arsenic Electron dot diagrams, sometimes called Lewis dot diagrams, were first used by Gilbert N. Lewis in These diagrams are used as a. Comprehensive information for the element Arsenic - As is provided by this page including scores of Atomic Structure of Arsenic Electron Dot Model.
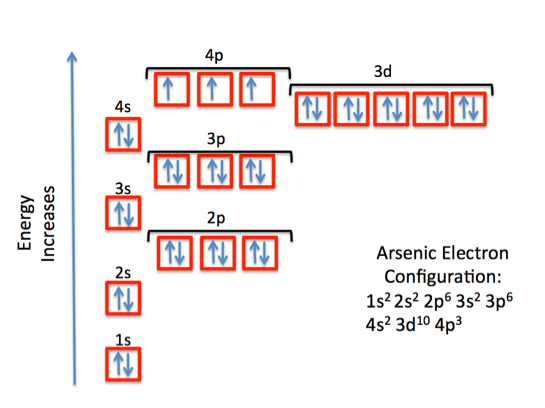
What Is The Correct Lewis Structure For Group 5A Element ... Thus, the electron configuration for arsenic is [Ar]4s 2 3d 10 4p 3. Before writing the electron - dot structure for arsenic, note that arsenic's ten 3d electrons are not in the highest principal energy level. How many electron dots does arsenic have?
Lewis Structure Calculator - MathAuditor To generate the Lewis dot structure, you have to follow the given steps: Find the total count of valence electrons to molecules. In this step, add the total count of valence electrons from all the atoms in a bit. Find the required count of electrons needed to make the atoms complete. After then, define the number of bonds in the given molecule.
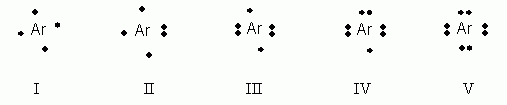
The electron dot diagram shows the arrangement of dots ... The electron dot diagram shows the arrangement of dots without identifying the element. ? with 2 dots above and 1 dot each right, below, left. Which element's symbol could replace the question mark in the diagram? boron (B) neon (Ne) rubidium (Rb) arsenic (As)
cadmium lewis dot structure - jardinefoods.com Silver tends to form ions with a charge of 1+, but the elements to the left and right of silver in the periodic table tend to form ions with 2+ charges. So that is the Lewis dot structure. Comprehensive information for the element Arsenic - As is provided by this page including scores of Atomic Structure of Arsenic Electron Dot Model.
How to Draw the Lewis Dot Structure for Arsenic (As ... A step-by-step explanation of how to draw the Arsenic (As) Lewis Dot Structure.For the ArsenicLewis structure use the periodic table to find the total number...
What is the correct Lewis structure for arsenic? | Socratic Arsenic is isoelectronic with nitrogen (they are both Group V elements), so there are 5 valence electrons. Look at the position of arsenic in the Periodic Table. It is under phosphorus, which is under nitrogen. These elements are thus isoelectronic, and their chemistry should be similar to a first approximation. Consider ammonia versus phosphine versus arsine, i.e. NH_3 versus PH_3 versus AsH_3.
How to draw AsF3 Lewis Structure? - Science Education and ... In the As F3 Lewis structure diagram, we always begin by introducing valence electrons from the central Arsenic atom (in step1). As a result, wrap around the central Arsenic atom's bond pair valence electrons first (see figure for step1). The Arsenic atom in the molecule gets only 8 electrons around its molecular structure.
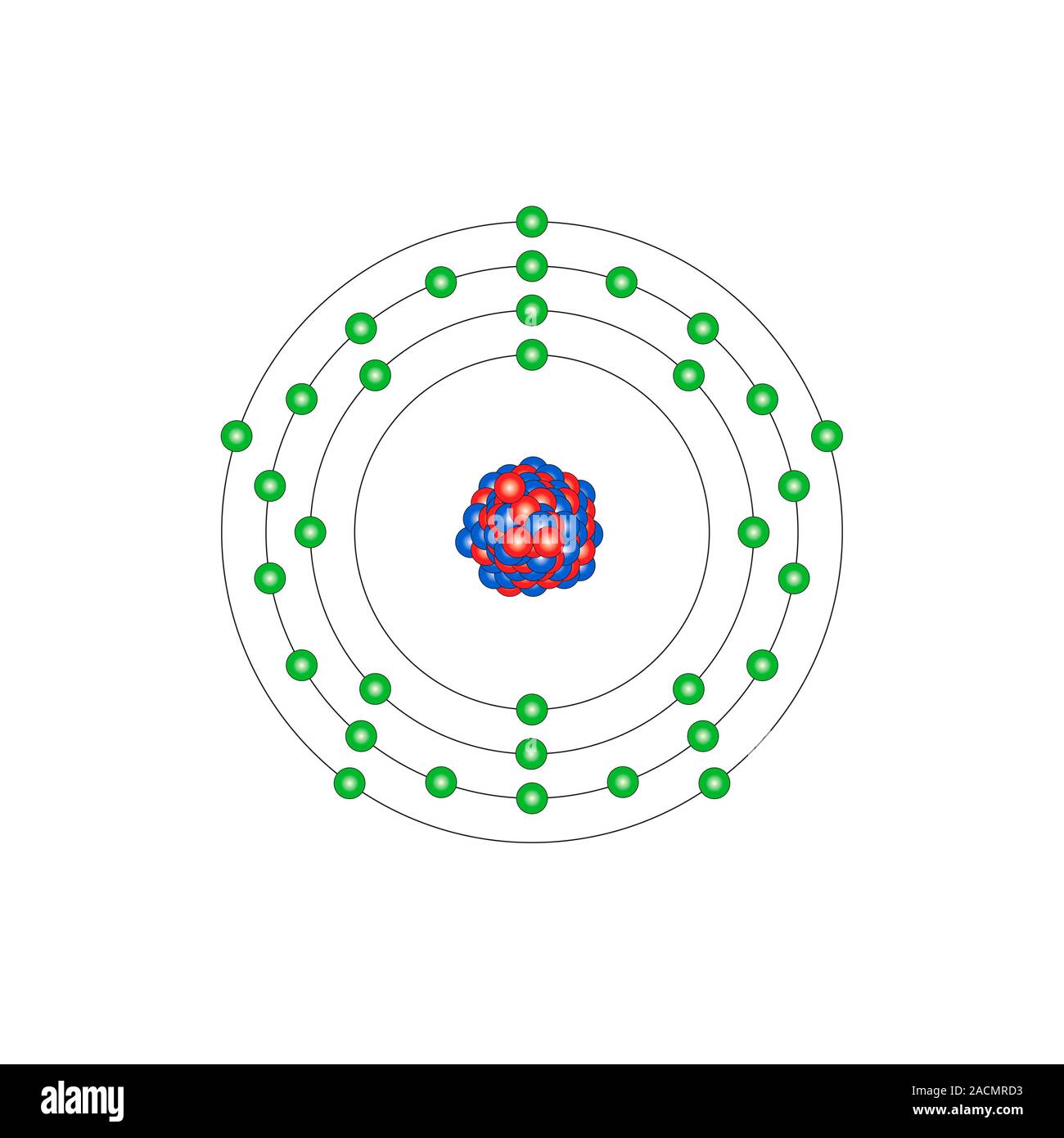
Lewis Dot Diagram For Arsenic - schematron.org DRAWING LEWIS DOT DIAGRAMS 1) Given a Bohr model, count the 2 Mg DRAWING ELECTRON DOT DIAGRAMS Draw the Dot Diagram for arsenic (As). Comprehensive information for the element Arsenic - As is provided by this page including scores of Atomic Structure of Arsenic Electron Dot Model. The left diagram shows a Lewis dot structure of sodium with.
What is the electron configuration for As? | Socratic By using the Aufbau diagram (shown below) we can determine the full electron configuration for a neutral arsenic atom. 1s22s22p63s23p63d104s24p3 The shorthand electron configuration, which uses the symbol for the noble gas in the previous period, in this case argon. [Ar]3d104s24p3 Aufbau Diagram Answer link
Lewis Dot Diagram For Arsenic Arsenic is isoelectronic with nitrogen (they are both Group V elements), so there are 5 valence electrons.A handy way to illustrate these valence electrons is to use Lewis diagrams, also called electron dot diagrams. These diagrams show the symbol of the element with as many dots around it as there are electrons in the outermost energy level.
Arsenic triiodide | AsI3 - PubChem Arsenic triiodide | AsI3 | CID 24575 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety ...
PLEASE HURRY WILL MARK BRAINLIEST !!!!!!!!!!!The electron ... The answer would be Arsenic (As). An electron-dot diagram is a graphical representation of the valence electrons of a certain element. The chemical symbol of an element placed in the middle and the valence electrons are represented by dots. The number of dots is equal to the number of valence electrons.
AsF3 Lewis Structure - How to Draw the Dot Structure for ... The Lewis structure for AsF 3 is similar to AsCl 3 structure. Since they are in the same Group on the periodic table they each have the same number of electrons their structures are similar. The Arsenic atom goes in the center of the Lewis structure since it is the least electronegative atom.
How to draw AsCl3 Lewis Structure? - Science Education and ... In the AsCl3 Lewis structure diagram, we always begin by introducing valence electrons from the central Arsenic atom (in step1). As a result, wrap around the central Arsenic atom's bond pair valence electrons first (see figure for step1). The Arsenic atom in the molecule gets only 8 electrons around its molecular structure.








![Arsenic acid]](https://www.degruyter.com/document/doi/00.0000/IUPAC.iupac.compound.234/asset/images/234.png)



















0 Response to "42 Arsenic Electron Dot Diagram"
Post a Comment