40 lewis dot diagram for o3
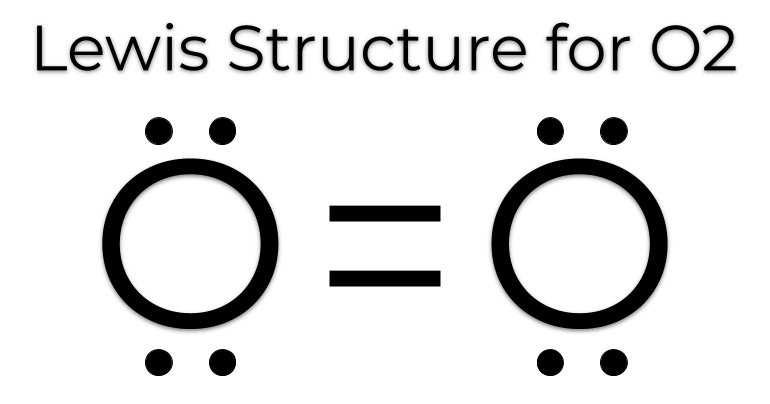
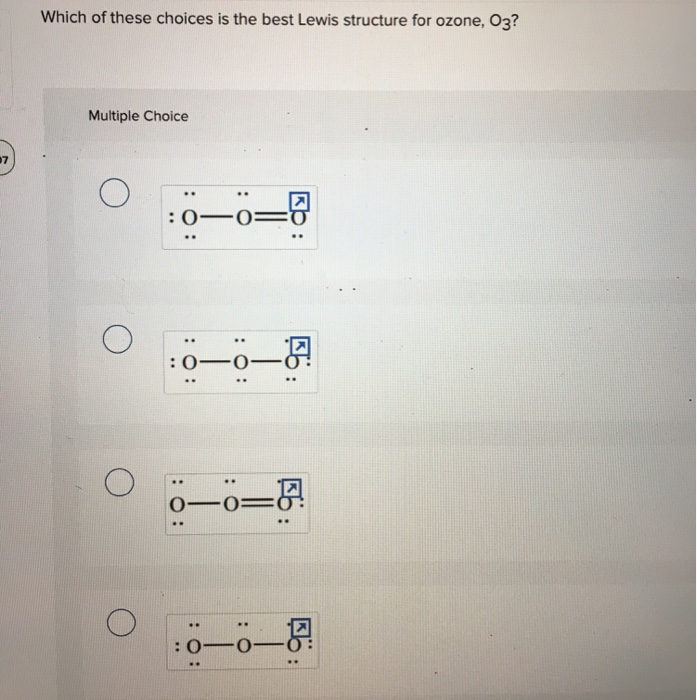
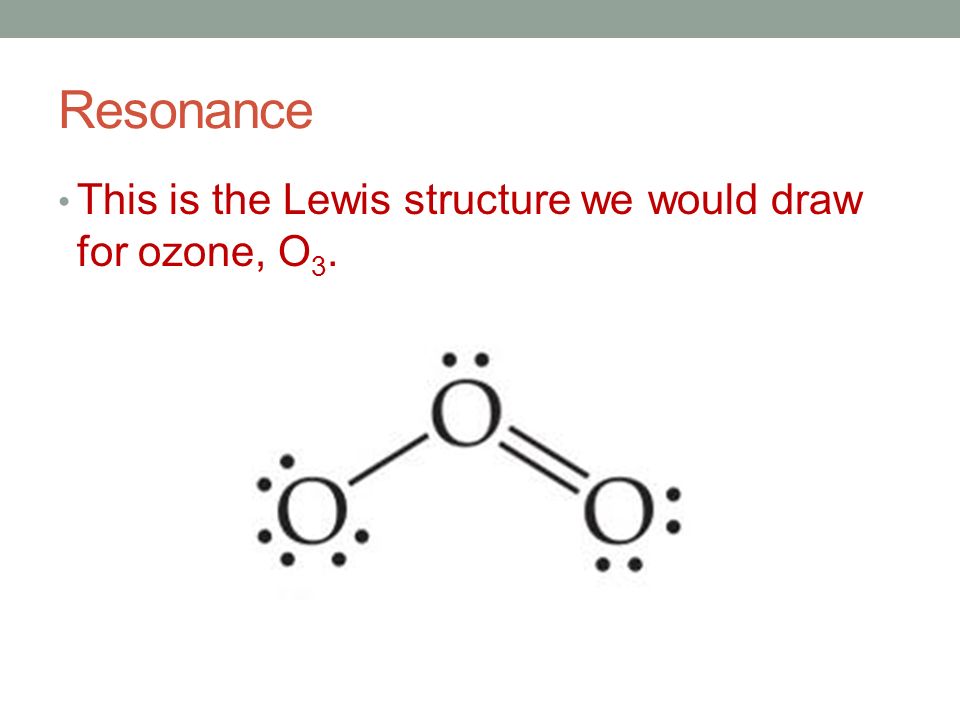
Ozone (O3) is an allotrope of oxygen and contains three oxygen atoms. In the lewis structure of ozone, there are one double bond and one single bond. Also, there are charges in two oxygen atoms in O 3 lewis structure. Lewis structure of O 3 can be drawn by starting from valence electrons of oxygen atoms in several steps. I quickly take you through how to draw the Lewis Structure of O3 (Ozone). I also go over the resonance, hybridization, shape and bond angle.
Click to get the latest Buzzing content. Sign up for your weekly dose of feel-good entertainment and movie content!

Lewis dot diagram for o3
We always make sure that writers follow all your instructions precisely. You can choose your academic level: high school, college/university, master's or pHD, and we will assign you a writer who can satisfactorily meet your professor's expectations. For the Lewis Structure for O 3 it is possible to draw it two different says (slightly different, but still important). These are called resonance structures. O 3 (Ozone) is important in the upper atomsphere since it blocks UV light that can be harmful to humans (for example: causing skin cancer). eq. 1.1) O + O 2 O 3 {\displaystyle {\ce {O + O2 -> O3}}} (eq. 1.2) NO 2 + O 2 → h ν NO + O 3 {\displaystyle {\ce {NO2 + O2 ->[h \nu] NO + O3}}} (eq. 1.3) NO + O 3 NO 2 + O 2 {\displaystyle {\ce {NO + O3 -> NO2 + O2}}} (eq. 1.4) In the upper atmosphere, the photodissociation of normally unreactive chlorofluorocarbons (CFCs) by solar ultraviolet radiation is an important …
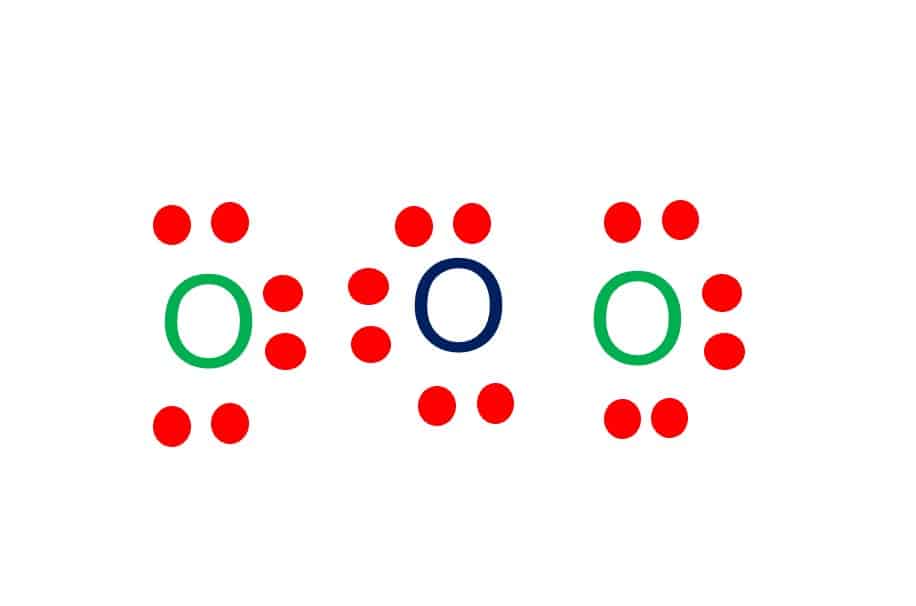
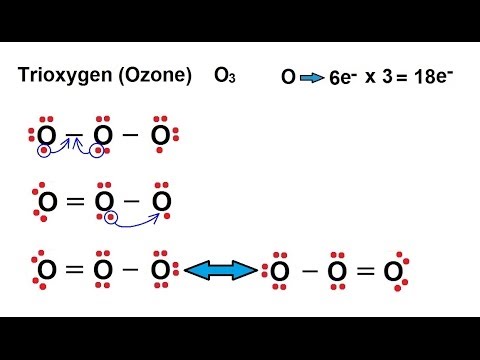
Lewis dot diagram for o3. The Lewis structure of ozone (O3) 1. Sum of valence electrons = (6*3) = 18 2. Drawing the bond connectivities: 3. Complete the octets of the atoms bonded ... Oct 31, 2018 · I was wondering why the Lewis structure for O3 is O-O=O ( where the Formal charge from left to right is -1,+1,0) (3 e- pairs. Consider the case of ozone O3 Lewis electron dot structures: 2 π electrons (pi electrons) in O3 and so 1 double bond must be added to the structure of Step 1.How to Draw a Lewis Structure Find the Total Number of ... VSEPR Theory Questions and Answers. Get help with your VSEPR theory homework. Access the answers to hundreds of VSEPR theory questions that are explained in a way that's easy for you to understand. Cambridge International AS and A Level Mathematics Mechanics. 370 Pages. Cambridge International AS and A Level Mathematics Mechanics
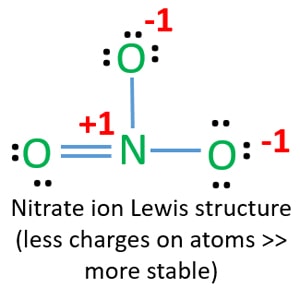
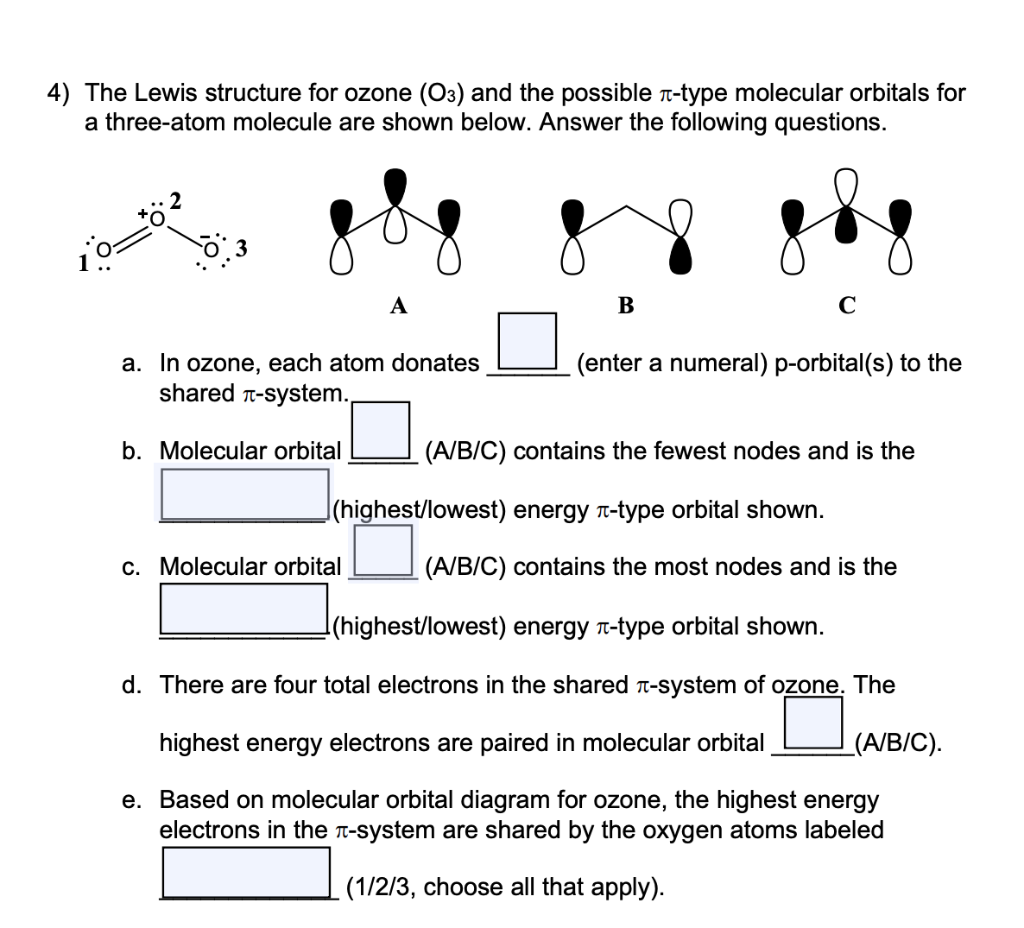
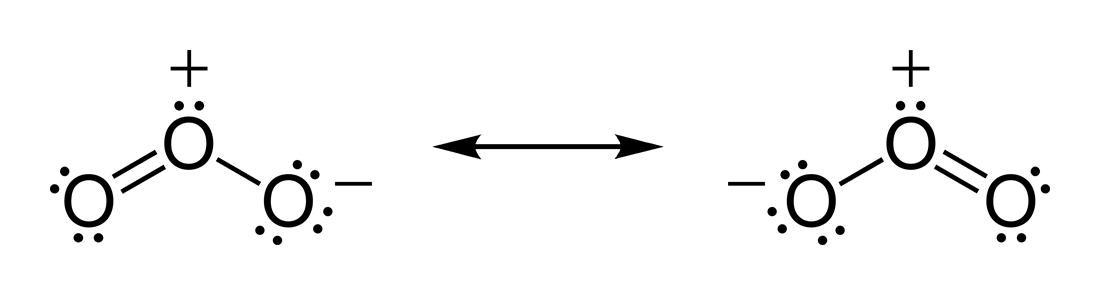
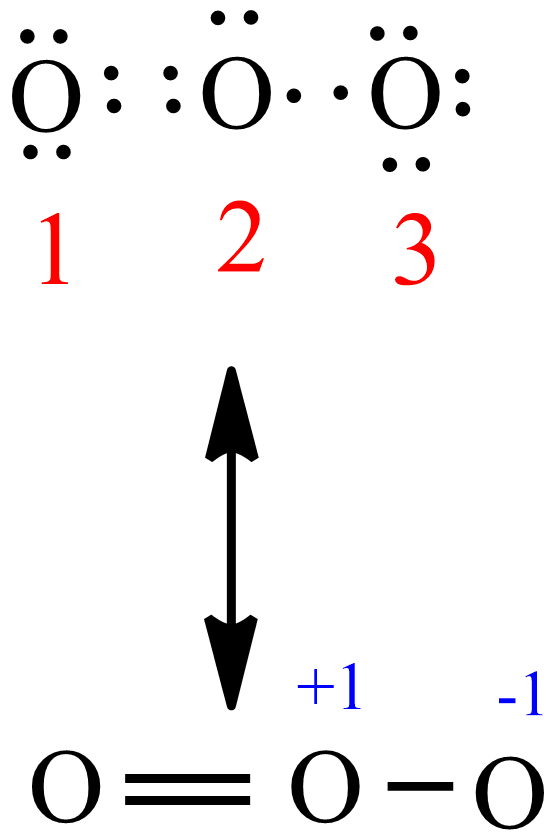
(c) In the box below, complete the Lewis electron-dot diagram for HCOOH. Show all bonding and nonbonding valence electrons. H. 2 2 2. NNH ()aq H O (l) H Explanation: Simple VESPER requires that we distribute 3 ×6 = 18 valence electrons across 3 centres: O = O+ − O−. From the left, O1, has TWO lone pairs; O2 has ONE lone pairs; and O3 has THREE lone pairs. And thus the formal charge of each oxygen atom ( 8e−,7e−,9e−) is 0, + 1, −1 respectively. Because there are THREE regions of ... Feb 02, 2022 · Lewis Structure of O3. Here, we will be dealing with ozone, the molecular formula is O3. The below discussion, therefore, will be based on finding out the Lewis Structure of O3. Ozone consists of three oxygen atoms. Oxygen belongs to group VI of the periodic table with an atomic no of 8. It thus has 6 valence electrons. Book of Organic Chemistry . Vollhardt Organic Chemistry Structure Function 6th txtbk.PDF
The central atom in the Lewis structure will have a charge of +1 and the atom forming a single bond will have -1 charge. O3 Hybridization Hybridization in chemistry means the hybridising of two or more atomic levels of the same or different energies to combine and give a new orbital. Your grades could look better! All our academic papers are written from scratch. All our clients are privileged to have all their academic papers written from scratch. A step-by-step explanation of how to draw the O3 Lewis Dot Structure (Ozone).For the O3 structure use the periodic table to find the total number of valence ... lewis o3 dot diagram structure ozone chem oxygen draw hi possible study guide atwood 1100 number bond allotrope . lewis dot o3 diagram structures . lewis structure o3 ozone dot formal stack slidesharefile charges chemistry exchange . oxygen o3 examples allotropes formulas lewis structure ozone study .
5.2.2022 · The Business Journals features local business news from 40-plus cities across the nation. We also provide tools to help businesses grow, network and hire.
General-Chemistry-1.pdf - Free ebook download as PDF File (.pdf), Text File (.txt) or view presentation slides online.
Lewis Structure of Al2O3 The concept of Lewis structure was first introduced by Gilbert N. Lewis in 1916. It is also known as the Lewis dot diagram or electron dot structure. It is the structural illustration of the position of the valence electrons, involved in the formation of a chemical bond, around the atoms inside a molecule.
eq. 1.1) O + O 2 O 3 {\displaystyle {\ce {O + O2 -> O3}}} (eq. 1.2) NO 2 + O 2 → h ν NO + O 3 {\displaystyle {\ce {NO2 + O2 ->[h \nu] NO + O3}}} (eq. 1.3) NO + O 3 NO 2 + O 2 {\displaystyle {\ce {NO + O3 -> NO2 + O2}}} (eq. 1.4) In the upper atmosphere, the photodissociation of normally unreactive chlorofluorocarbons (CFCs) by solar ultraviolet radiation is an important …
For the Lewis Structure for O 3 it is possible to draw it two different says (slightly different, but still important). These are called resonance structures. O 3 (Ozone) is important in the upper atomsphere since it blocks UV light that can be harmful to humans (for example: causing skin cancer).
We always make sure that writers follow all your instructions precisely. You can choose your academic level: high school, college/university, master's or pHD, and we will assign you a writer who can satisfactorily meet your professor's expectations.























![High School Chem]: Is this the correct Lewis Structure for ...](https://preview.redd.it/pzmn9pdp2io41.jpg?auto=webp&s=9778348f6a62c80dcdd0eb84a10bbcb32da471bf)
0 Response to "40 lewis dot diagram for o3"
Post a Comment