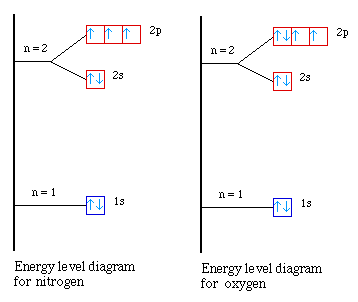
38 energy level diagram for oxygen
Part of a series of articles about. Quantum mechanics. Schrödinger equation. Introduction. Glossary. History. v. t. e. A quantum mechanical system or particle that is bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. Energy Levels of Neutral Oxygen ( O I ). Configuration. Term. J. Level(cm-1). Ref. 2s22p4.
Oxygen_energy_level_diagram_-_fr.jpg (315 × 321 pixels, file size: 10 KB, MIME type: image/jpeg). It is recommended to name the SVG file "Oxygen energy level diagram - fr.svg" - then the template Vector version available (or Vva) does not need the new image name parameter.

Energy level diagram for oxygen
The first ten molecular orbitals may be arranged in order of energy as follow Diagram for O2+ is wrong because 2p atomic orbital of 2nd O atom will have only 3 e-. Reply. Mrs Shilpi Nagpal says. September 26, 2018 at 11:06 am. I am asked to draw an energy level diagram that shows the relationship between the energy quantities involved in the problem. What I did was I calculated the standard enthalpy change of the reaction first (-43.16 kJ/mol-rxn) and then drew the diagram below. Did I draw the diagram correctly? [Energy Diagram I drew](https://preview.redd.it/yl8e8l4byg081.jpg?width=1280&format=pjpg&auto=webp&s=2f24a33862f2e8c7dace0351ce643c3a2666e2ac) [The Question](https://preview.redd.it/fry8myexxg0... 7. Consider the orbital diagram for oxygen in Model 2. 14. Below are three answers generated by students in response to the prompt: "Provide an orbital energy level diagram for the ground state of a nitrogen atom."
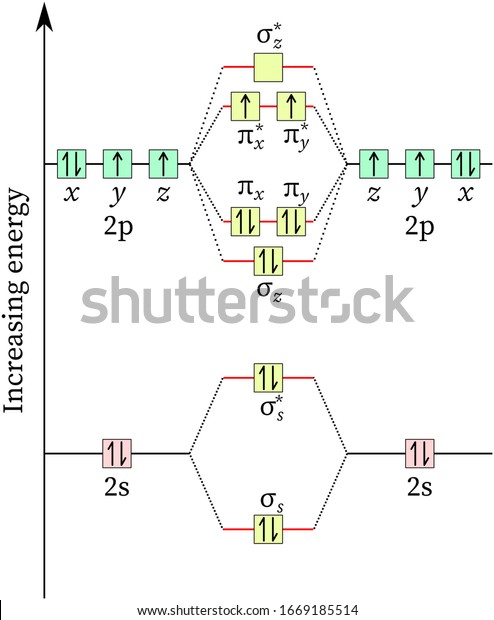
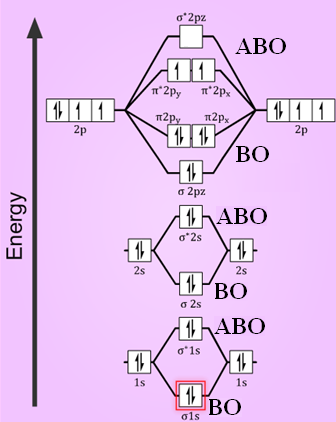
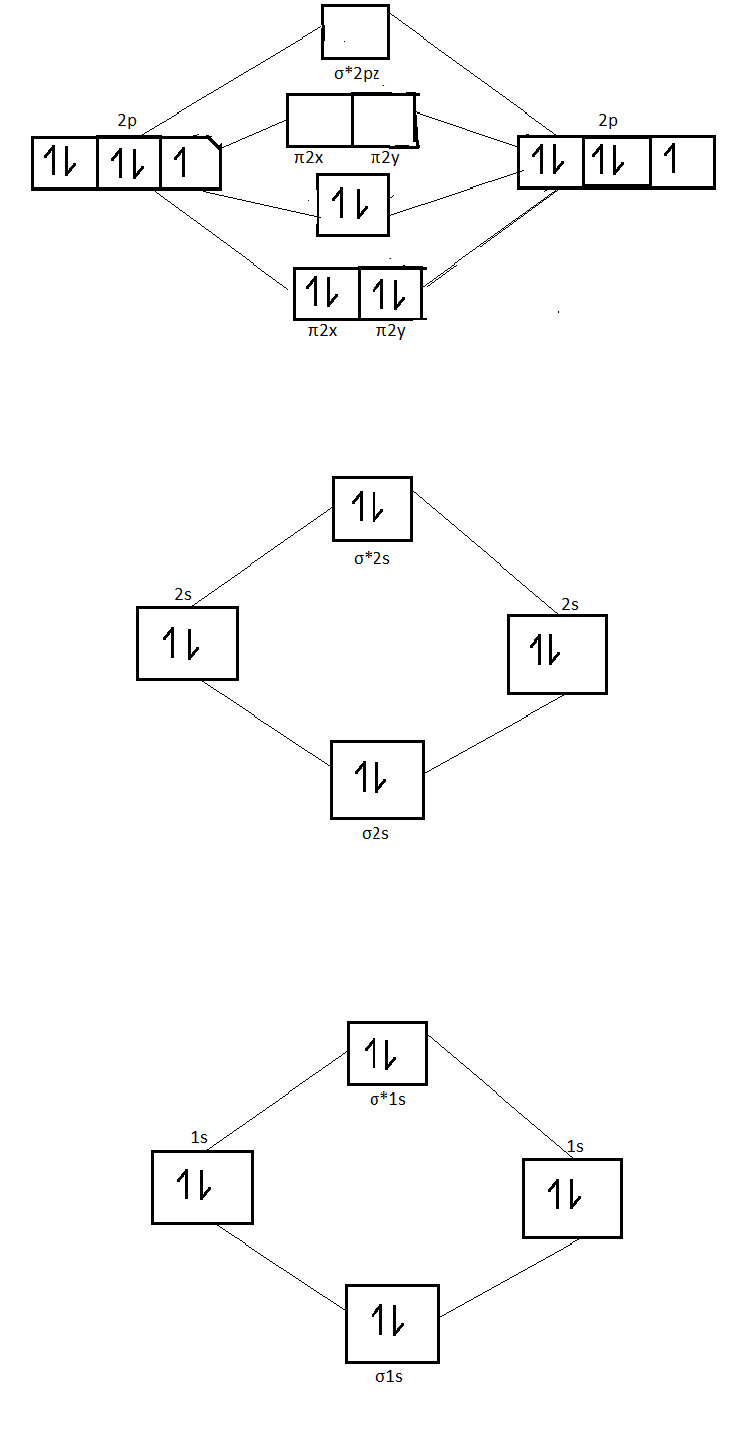
Energy level diagram for oxygen. A family member showed 97-98 over the past few days and now shows around 94-95. Should I be concerned? • Because the energy of the two electrons is lower than the energy of the individual atoms, the molecule is stable. Figure 9.26: (a) The molecular orbital energy-level diagram for the H2 molecule. (b) The shapes of the molecular orbitals are obtained by squaring the wave functions for MO1 and MO2. Atomic number of oxygen = 8. Electronic configuration of oxygen = 1s22s22p4. When two oxygen atoms combines, the molecular orbital energy level diagram is as shown in the figure. Alternative energy level diagram for BeH. HOMO - 3σ low energy Oxygen orbitals makes 2σ Æ mainly O pz Æ in 3σ mainly C pz. Some anti-bonding mixes in. due to sp mixing.
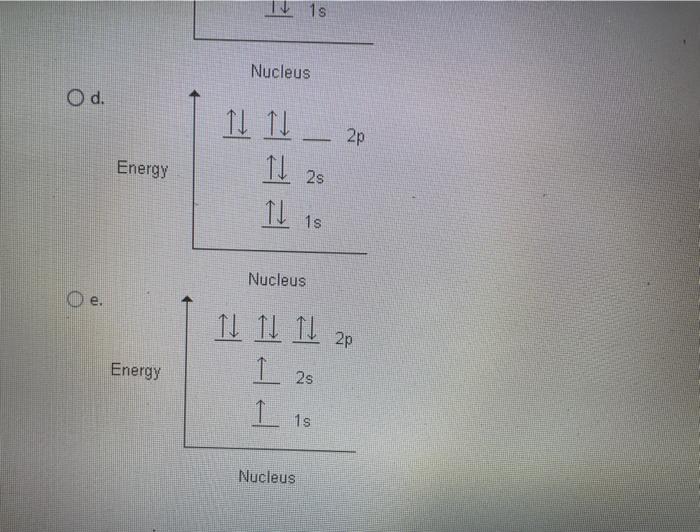
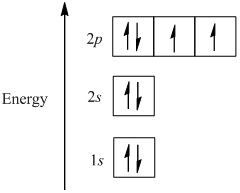
first energy level:2 second energy level: 8 third energy level: 18 fourth energy level: 15 fith energy level: 1. Because the electron configuration for oxygen is 1s2 2s2 2p4, therefore 2p being the greatest or highest energy level. The molecular orbital energy level diagram of H2 molecule is given in Fig.. The bond order of H2 molecule can be calculated as follows. The electronic configuration of oxygen (Z = 8) in the ground state is 1s22s22p4. Each oxygen atom has 8 electrons, hence, in O2 molecule there are 16 electrons. Take the valency of oxygen to be 2.(1) SO3(2) N2O3(3) SnO2. In using the energy level diagram, remember two things: Electrons fill the lowest vacant energy levels first. When there's more than one subshell at a...In the electron config-uration for oxygen, 1s22s22p4, energy level 1 is filled, and there are two electrons in the 2s orbital and four electrons in...
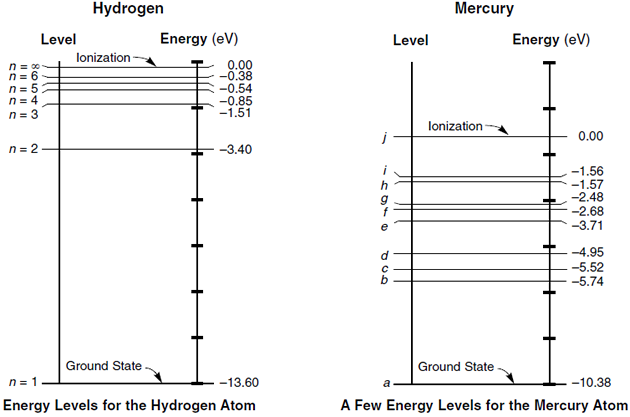
Help pls The oxygen-oxygen distance of 120.7 pm in O2 is comparable to the distance expected for a double bond. Molecular orbital energy level diagrams for these second-row heteronuclear diatomics can be drawn rather easily by modifying the homonuclear pattern slightly (as described for O2, Figure 2-4). It's often helpful to draw a diagram showing the energy levels for the particular element you're interested in. The diagram for hydrogen is shown above. The n = 1 state is known as the ground state, while higher n states are known as excited states. If the electron in the atom makes a transition from a... Jablonski diagram for the excited states of molecular oxygen. When the excitation energy of singlet oxygen is taken into consideration the values of E˚ (1O2/ O2•) are 0.34 V in dimethylformamide and 0.79 V in water.
This WebElements periodic table page contains properties of free atoms for the element oxygen. J. C. Fuggle and N. Mårtensson, "Core-Level Binding Energies in Metals," J. Electron Spectrosc.
Energy Level Diagram - Different Energy Shells Around the. 9 hours ago Below is a blank energy level diagram which helps you depict electrons for any Write the Electronic configuration, Energy level diagram for the molecular orbitals of Oxygen molecule (O2). Calculate its bond order and give...
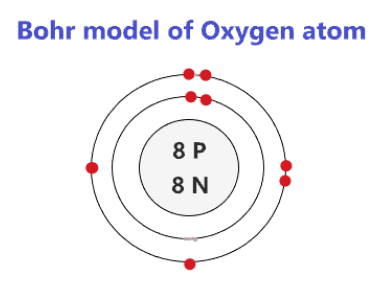
O (Oxygen) is an element with position number 8 in the periodic table. Located in the II period. Melting point: -218.4 ℃. Density: 0.00133 g/cm3. Below is the electronic diagram of the Oxygen atom. Distribution of electrons over energy levels in the O atom 1-st level (K): 2 2-st level (L): 6.
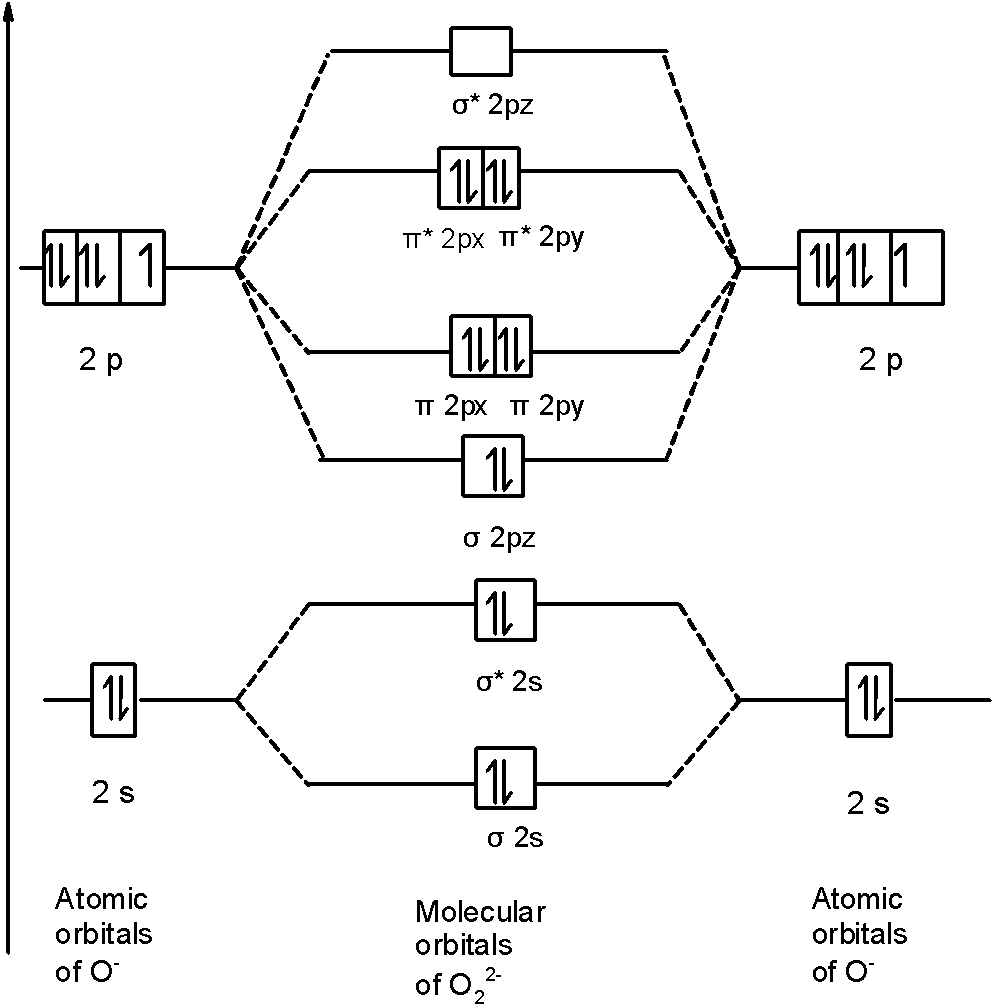
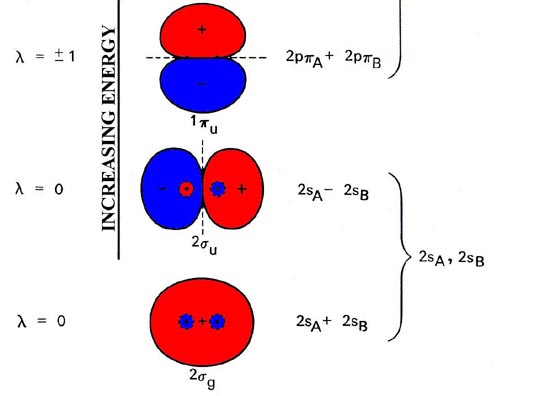
In completing the molecular orbital energy level diagram for oxygen, we discover that we must decide whether to pair The molecular orbital energy level diagrams in Figures 9.5 to 9.7 cast a new light on this analysis. Note that, in each case, the number of bonding electrons in these molecules is eight.
For U ) 1.23 V, the free-energy diagram for the case of an oxygen coverage of 1 / 2 is included. from publication: Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode | We present a method for calculating the stability of reaction intermediates of electrochemical processes on the basis...
2-17.—Energy-level diagram for the neutral oxygen atom. Figure 11.6 Molecular orbital energy level diagrams computed for iron octahedrally coordinated to oxygen. Left divalent iron in the [Fe06]-1° cluster (based on Sherman, 1991) right trivalent iron in the [Fe06]-9 cluster (from Sherman, 1985a).
An energy level diagram is a diagram that shows the energies of the reactants, the transition state(s) and the products of the reaction with time. The transition state is a stage during the reaction at which chemical bonds are partially broken and formed. The transition state is very unstable - it cannot be...
The above diagram explains the molecular orbital energy level diagram for molecules of Oxygen and other heavier elements. The electronic configuration of oxygen (Z=8) in the ground state is 1s22s22p4. Each oxygen atom has 8 electrons; hence in O22 is as follows molecule there are 16...
From the molecular orbital diagram, we observe that oxygen has two unpaired electrons which is consist with the paramagnetic nature of oxygen. To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels in the diagram) one at a time until you have used...
An element's electron configuration can be represented using energy level diagrams, or Aufbau diagrams. The Aufbau principle (from the German As another example, oxygen has 8 electrons. The electron configuration can be written as 1s22s22p4. The orbital diagram is drawn as follows: the first...
Figure 8: Molecular orbital energy-level diagrams for (A) beryllium hydride, BeH2, with linear shape, and (B) water, H2O, with bent shape. The molecular orbitals are labeled to reflect the atomic orbitals from which they are composed as well as their symmetry properties.
Energy level diagram of Co 2+ in a tetrahedral and octahedral ligand field. 17.53 exhibits 20 lines from the various oxygens and these are identified in the caption. The use of aligned grains considerably increased the resolution of this spectrum, indicating a considerable amount of anisotropy.
Energy levels are fixed distances where electrons are rotating around the nucleus with definite But difficult for readers to remember the electron energy levels diagram for many electronic Electronic configuration of group-16 elements. Oxygen, sulfur, selenium, tellurium, and polonium in the periodic...
Note: In the energy level diagrams, the electrons are spread out evenly in the level. Some books show them spread out this way and some show them in pairs. The pairing of electrons is meant to represent that electrons are in separate orbitals within each energy level. At the middle school level...
Energy level diagram of oxygen from class 11th chemistryHope you all like this video Thank you for watching the video#energy_level_diagram#anandsirclasses.
7. Consider the orbital diagram for oxygen in Model 2. 14. Below are three answers generated by students in response to the prompt: "Provide an orbital energy level diagram for the ground state of a nitrogen atom."
I am asked to draw an energy level diagram that shows the relationship between the energy quantities involved in the problem. What I did was I calculated the standard enthalpy change of the reaction first (-43.16 kJ/mol-rxn) and then drew the diagram below. Did I draw the diagram correctly? [Energy Diagram I drew](https://preview.redd.it/yl8e8l4byg081.jpg?width=1280&format=pjpg&auto=webp&s=2f24a33862f2e8c7dace0351ce643c3a2666e2ac) [The Question](https://preview.redd.it/fry8myexxg0...
The first ten molecular orbitals may be arranged in order of energy as follow Diagram for O2+ is wrong because 2p atomic orbital of 2nd O atom will have only 3 e-. Reply. Mrs Shilpi Nagpal says. September 26, 2018 at 11:06 am.




















0 Response to "38 energy level diagram for oxygen"
Post a Comment