37 molecular orbital diagram for n2 2-
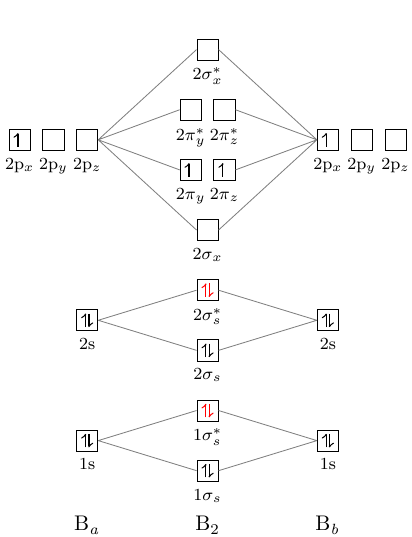
So, it will have some net magnetic moment. Atomic number of nitrogen is seven. Therefore in ${N_2}$ there are a total fourteen electrons. But $N_2^ - $ has one electron more than that of nitrogen molecule. So, total electrons are fifteen. Molecular orbital diagram of $N_2^ - $ is shown below: Why is the MO diagram different for N2 and N2-? I have been taught that the MO diagram is different for molecules with 14 or less electrons than the one used for molecules with 15 or more electrons. For $\\ce {N2}$ the orbitals in increasing energy are: because it has 14 electrons. For $\\ce {N2-}$ there are 15 electrons.
Molecular Orbital Diagram of N2 Molecular orbitals exist in molecules where each molecule has its electron configuration in terms of a sigma bond and pi bond. According to molecular orbital theory, it tells about magnetic nature, stability order, and the number of bonds in a molecule.

Molecular orbital diagram for n2 2-
Question: Draw the molecular orbital diagram for N2^2- Determine the bond order and if it is stable or not. ***Please explain how you determine if it is stable through the bond order. Thank you · This problem has been solved! ... Draw the molecular orbital diagram for N2^2- Determine the bond ... Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ... Aug 07, 2021 · Nitrogen atom has electronic configuration 1s2, 2s2, 2p3. Two p-atomic orbitals (one from each nitrogen) atom combine to form two molecular orbitals, the bonding molecular orbital σ2px and antibonding molecular orbital σ*2px. The other four p-atomic orbitals (two from each nitrogen) atom combine to give four molecular orbitals, two bonding molecular orbitals i.e. π2py and π2pz, while two antibonding molecular orbitals i.e. π*2py and π*2pz.
Molecular orbital diagram for n2 2-. March 7, 2018 - Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams. Question: Use Molecular Orbital (MO) Diagrams To Rank N22+, N2, And N2? In Order Of Increasing Bond Order, Bond Energy, And Bond Length. Fill Order For MO: ?2s, ?*2s, ?2p = ?2p, ?2p , ?*2p = ?*2p, ?*2p Bond Order: N2 < N2? < N22+ ; Bond Energy: N2 < N22+ < N2 ? Explain why the relative energy levels diagrams for Li2, Be2, B2, C2, N2 are different The molecular orbital theory of Li2 to F2 gives a graphical explanation. Energy level diagram for Molecular orbitals. May 25, By Mrs Shilpi Nagpal 9 . It is paramagnetic in nature. 6)Li2. Molecular orbital energy level of Li2.Molecular orbitals of Li 2, Be 2 ... Draw the molecular orbital diagram for N2. Label all of the atomic orbitals and molecular orbitals and put the correct number of electrons in. You do not need to draw the shapes of any of the orbitals. a) MO diagram b) Based on your MO diagram, is N2 diamagnetic or paramagnetic? c) Calculate the bond order for N2.
Write the molecular orbital diagram of N2+ and calculate their bond order why nitrogen have different structure of molecular orbital theory An atomic orbital is monocentric while a molecular orbital is polycentric. You want to lead a successful business, launch a breakthrough innovation, or even change the way we do business in the 21st century. KPU's Melville School of Business gives you the real-world skills, practical know-how, and professional connections you need to achieve what is possible. Sp mixing - https://youtu.be/p3ME8DYAih4Follow me on instagram-https://www.instagram.com/trickychemistrysuman/?hl=enFollow me on facebook page-https://lm.fac A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) N2 Molecular Orbital Diagram. N2 molecular orbital energy level diagram picture, is usually depicted by a diatomic molecules chapter learn consider the molecular orbital electron configuration notation to a molecular orbitals diagrams web the molecular orbital energy level ...
Nov 02, 2015 · If we build the MO diagram for "N"_2, it looks like this: First though, notice that the p orbitals are supposed to be degenerate. They weren't drawn that way on this diagram, but they should be. Anyways, for the electron configurations, you would use a notation like the above. g means "gerade", or even symmetry upon inversion, and u means "ungerade", or odd symmetry upon inversion. It's not ... Write the molecular orbital diagram of N2+ and calculate their bond order why nitrogen have different structure of molecular orbital theory An atomic orbital is monocentric while a molecular orbital is polycentric. Explain What is the relationship between bond order and the dissociation energy of a molecule? ... Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams. There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc).One is for the elements up to Nitrogen. The other is for AFTER nitrogen (start...
Draw the molecular orbital diagram of N2 and calculate the bond order. Answer: The bond order shows the number of chemical bonds present between a pair of atoms. The Bond Order Formula can be defined as half of the difference between the number of electrons in bonding orbitals and antibonding orbitals. Bond order formula is given as below.
Interactive video lesson plan for: MO Diagram for N2+ (Molecular Orbital) Activity overview: There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc).
4 Lecture 2 Pi bond (π): bonding molecular orbital -The bonding electron density lies above and below, or in front and in back of the bonding axis, with no electron directly on the bonding axis, since 2p orbitals do not have any electron density at the nucleus.
Molecular orbital (MO) diagram for N2 and N2^-Ask Question Asked 6 years, 5 months ago. Active 4 years ago. Viewed 119k times 25 8 $\begingroup$ I have been taught that the MO diagram is different for molecules with 14 or less electrons than the one used for molecules with 15 or more electrons. ... Now note that even in this advanced molecular ...
Click here👆to get an answer to your question ️ Use the molecular orbital energy level diagram to show that N2 would be expected to have a triple bond, F2 , a single bond and Ne2 , no bond.
November 9, 2015 - 12/20: Andy's paper on supported nanocrystal catalysts is published in Chem. Mater.! 10/20: Our joint paper with the Klimov group using ALD to produce CMOS circuit elements from CIS quantum dot films is published in Nature Comms. 9/20: Our paper with Adam Moule's group on electron tomography ...
Fill these electrons in the molecular orbitals that are shown in the following MO diagram by following Hund's rule. Hund's rule states that if two or more orbitals of equal energy are available, electrons will occupy them singly before pairing. MO diagram ofN2 is as follows: σ2px π2py 1-4-t 2 p -. . o2p, .. T2p n2p...
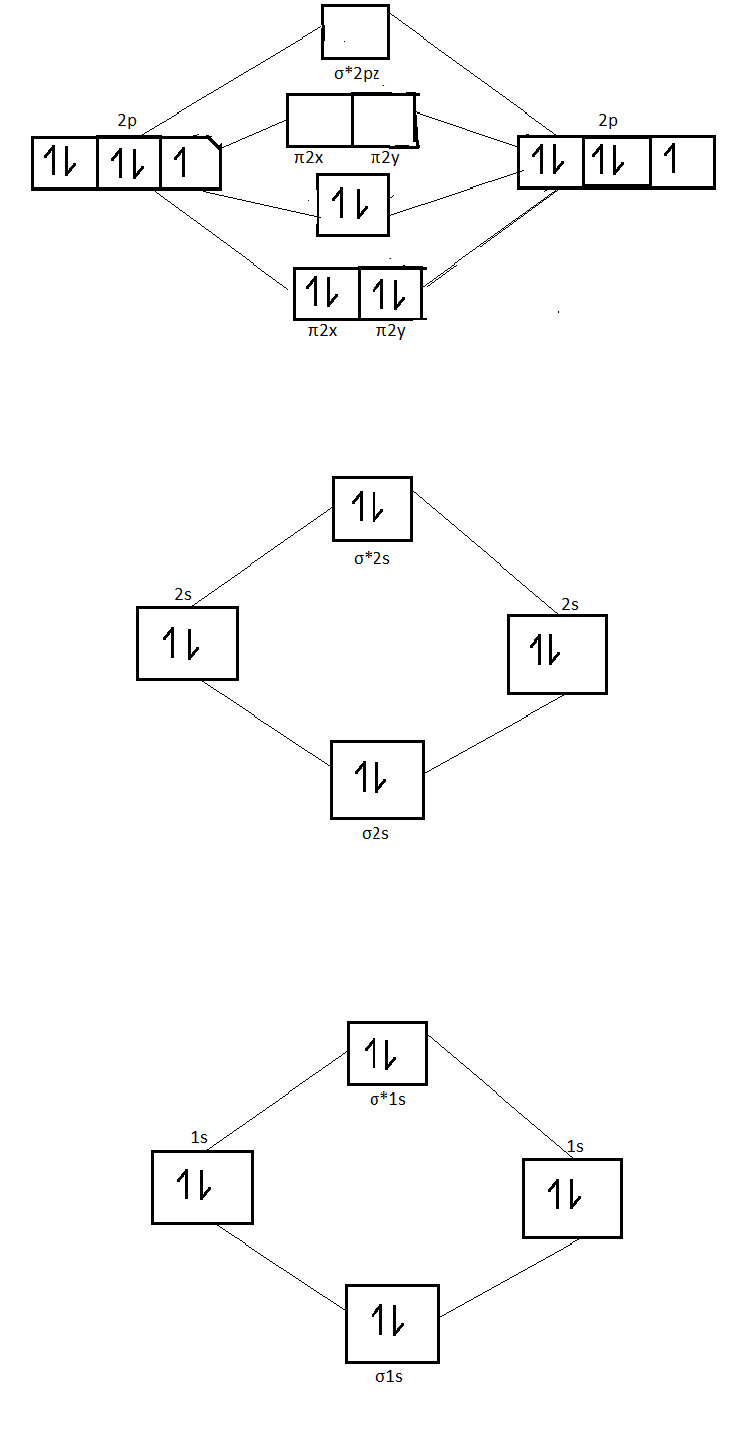
Here is the full molecular orbital diagram for N 2. Now we add the 10 electrons, 5 from each nitrogen atom. Note that the bottom sigma symmetry orbital is strongly bonding, the top one is strongly antibonding, and the 2 in the middle are only weakly bonding and antibonding, respectively. The 2 and 3 orbitals correspond to the non-bonding ...
Feb 03, 2019 · The diagram above is the molecular.N2 molecular orbital energy level diagram picture, is usually depicted by a diatomic molecules chapter learn consider the molecular orbital electron configuration notation to a molecular orbitals diagrams web the molecular orbital energy level structures can construct the molecular orbital energy level the energy than the atomic and form.
This video discusses how to draw the molecular orbital (MO) diagram for the N2 (nitrogen) molecule. The bond order of the nitrogen molecule is also calculate...
05/02/2015 · In the MO diagram of $\ce{N2^2-}$, does s-p mixing happen to a significant degree to change the ordering of orbitals - $\pi_\mathrm{2p}$ orbital below $\sigma_\mathrm{2p}$? As it is isoelectronic with oxygen, I think that the s-p mixing shouldn't change the ordering. molecular-orbital-theory. Share. Improve this question. Follow edited Mar 6 '17 at 21:11. EJC. asked Feb …
Thus, only 26% of σ*N(2p)N(2p) maps to valence shell NAOs, and the rest to n=3 or n=4 NAOs · Besides the shortfalls in the total contributions to MOs, the Table shows also that each NAO is not wholly accounted for. This is because the rest maps to even higher virtual MOs ... The σ orbitals (black in the Energy Level Diagram...
True or false: Boron contains 2s22p1 valence electrons, so only one p orbital is needed to form molecular orbitals. What charge would be needed on F2 to generate an ion with a bond order of 2? Predict whether the MO diagram for S2 would show s-p mixing or not. Explain why N22+ is diamagnetic, ...
Let me explain the molecular orbital diagram of N2 using its diagram. one atom of nitrogen has 7 electrons so a N2 molecule will have 14 electrons so first 2 electrons go in 1s sigma bond next 2 in 1s sigma anti bond orbital next 2 in 2s sigma bond orbital next 2 in 2s sigma anti bond orbital
In the molecular orbital diagram for the molecular ion, N 2 + , the number of electrons in the σ 2 p molecular orbital is: Hard. View solution. >. What is Molecular Orbital Theory. With the help of energy levels homonuclear diatomic orbitals, arrange the following species in increasing order of stability O 2 2 − , O 2 −, O 2 , O 2 +.
How many valence electrons are in this molecule? 2. Complete the MO diagram for the valence electrons in N22+. empty / ? / ? / ? / ? ? / ? ? / ? ? empty / ? / ? 3. Which is the highest occupied molecular orbital(s) (HOMO)? (i.e., Which orbital containing electrons has the highest energy?)
On a very general basis, electrons are not assigned to individual bonds between atoms, but they move under the influence of the nuclei in the whole molecule. Molecular orbital theory is a method for describing the electronic structure of the molecule. Now, let us draw the molecular orbital diagram of ${N_2}$ .
23/07/2020 · the pi(2p) bonding orbitals are LOWER than the sigma(2p) bonding orbitals.N2(2-) has a bonding order of 2, which predicts that there will be a stable double ...
Tagged as: #digitalkemistry, Bond order of Nitrogen molecule, chemical bonding, chemical bonding and shapes of molecules, chemistry, class 11 chemistry molecular orbital theory, class 11 chemistry molecular orbital theory notes, class 11 chemistry notes, digital kemistry, How do you draw a molecular orbital diagram, How do you find the bond ...
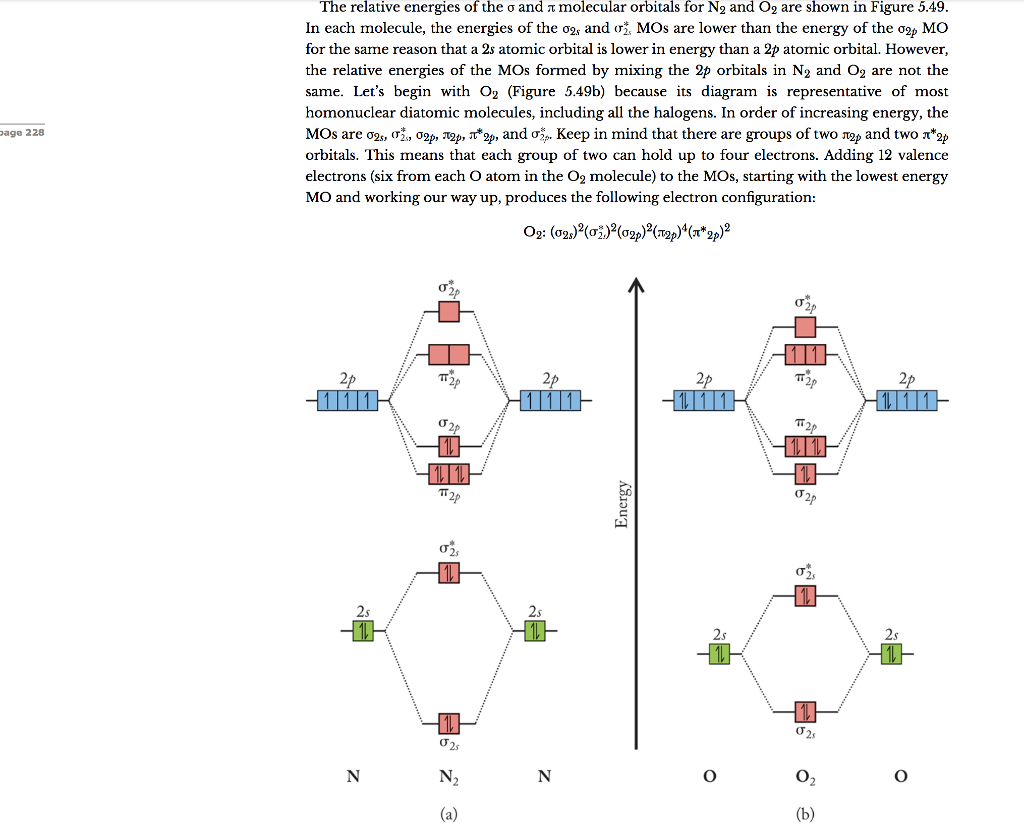
Answer (1 of 3): Here is the MO diagram for O₂: Whilst this is the MO diagram for N₂: If we compare such diagrams for the diatomic molecules on the Second Period (Li₂, Be₂, B₂, C₂, N₂, O₂, and F₂), the resulting pattern looks like this: When it comes to O₂ and N₂, I think there are two things ...
The unhybridized 2p1 orbital lies perpendicular to the three hybridised orbitals. Representation of sp 2 hybridization sp 2 hybridization is also known as trigonal hybridisation. Each sp 1 hybrid orbital has s-character and The molecular orbital structure of ethylene: In ethene molecule, each carbon atom undergoes sp 2 hybridisation.
Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular…
Draw the molecular orbital diagram of . N_2. molecule and write its molecular orbital configuration. Calculate the bond order and discuss the extra stability and diamagnetic nature of the molecule. 644038739. 300+ 7.3 k+. 08:34.
April 14, 2017 - NATURAL BOND ORBITALS (Summary): Principal Delocalizations NBO Occupancy Energy (geminal,vicinal,remote) =============================================================================== Molecular unit 1 (N2) 1. BD ( 1) N 1- N 2 2.00000 0.18488 2. BD ( 2) N 1- N 2 2.00000 -0.43475 10(g),42(g)
Click here👆to get an answer to your question ✍️ Draw the molecular orbital diagram of N2 , N2 + N2 - . Write their electronic configuration, find the bond order and predict their magnetic behaviour. Arrange the above in increasing order of bond length.
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.The Hydrogen Molecule Ion H2+Molecular Orbital Diagrams of Diatomic Molecules - Chem
Give the molecular orbital diagram of O_2 molecule and calculate its bond order. Molecular Orbital Theory||Energy Level Diagrams Molecular||Filling OF Electrons in Orbitals||Bond Or...
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation
Aug 07, 2021 · Nitrogen atom has electronic configuration 1s2, 2s2, 2p3. Two p-atomic orbitals (one from each nitrogen) atom combine to form two molecular orbitals, the bonding molecular orbital σ2px and antibonding molecular orbital σ*2px. The other four p-atomic orbitals (two from each nitrogen) atom combine to give four molecular orbitals, two bonding molecular orbitals i.e. π2py and π2pz, while two antibonding molecular orbitals i.e. π*2py and π*2pz.
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
Question: Draw the molecular orbital diagram for N2^2- Determine the bond order and if it is stable or not. ***Please explain how you determine if it is stable through the bond order. Thank you · This problem has been solved! ... Draw the molecular orbital diagram for N2^2- Determine the bond ...















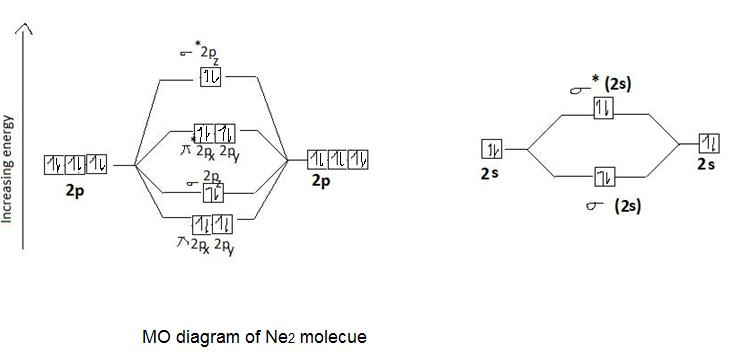





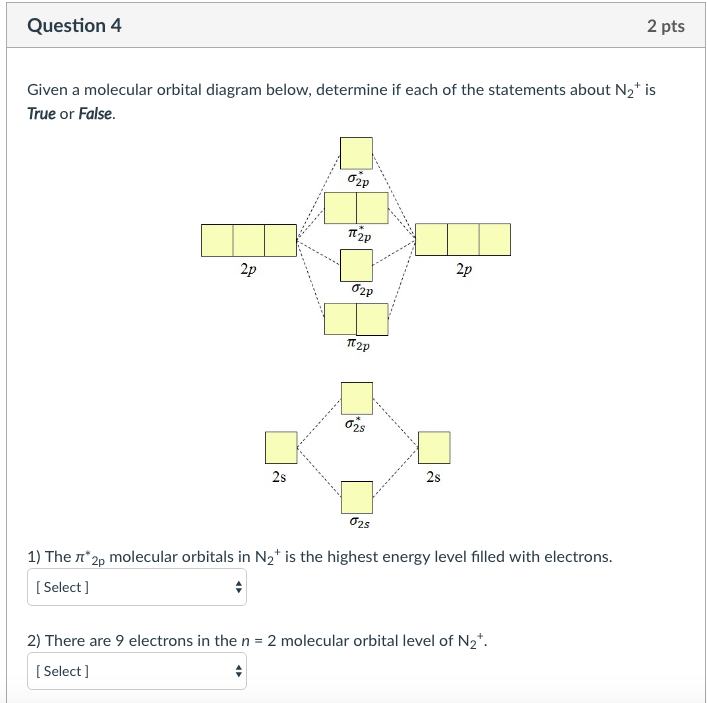

0 Response to "37 molecular orbital diagram for n2 2-"
Post a Comment