41 orbital diagram for ti2+
What is the electron configuration of Ti plus4? Wiki User. ∙ 2008-11-17 20:28:16. See Answer. Best Answer. Copy. The base expression (ground state; Ti0) is [Ar]3d24s2 so removal of 4 electrons ... ["Kr"]4d^10 Your starting point here will be the electron configuration of a neutral cadmium atom. Cadmium, "Cd", is located in period 5, group 12 of the periodic table and has an atomic number equal to 48. This means that a neutral cadmium atom will have a total of 48 electrons surrounding its nucleus. This also tells you that the "Cd"^(2+) cation, which has two electrons less than the ...
Orbital diagram for Sodium Now lets try one with ions! Electron configuration for N3-Because it has a charge of -3, there are three extra electrons to deal with. Normally, nitrogen has 7 electrons. But because of its charge, we have to add 3 more. If it has 10 electrons, write it out until you run out of electrons. 1s2 2s2 2p6

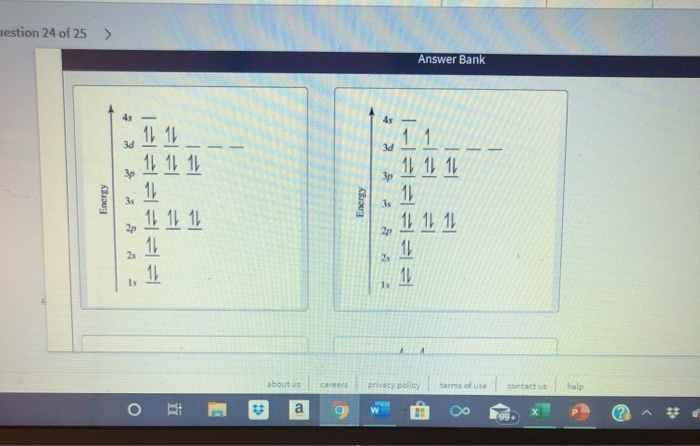
Orbital diagram for ti2+
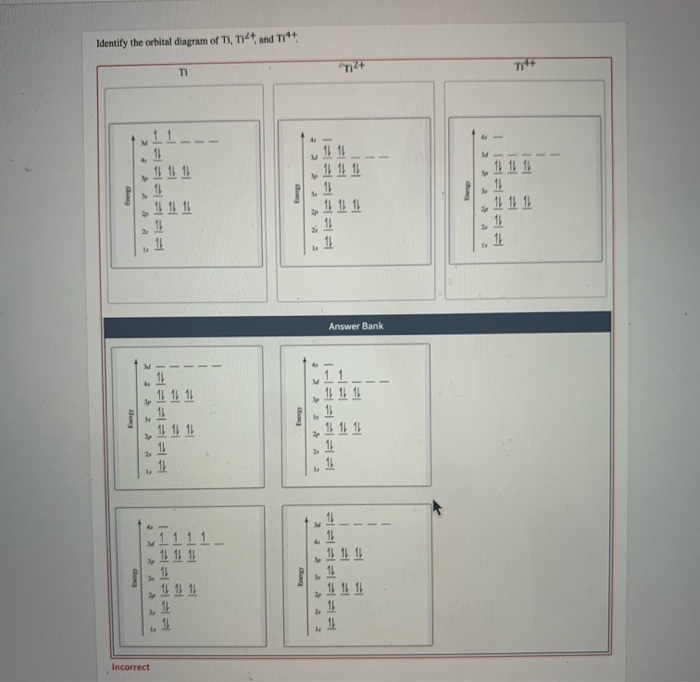
Orbital Diagram for Ti2. electron configuration for the titanium ion ti2 this video shows you how to write the electron configuration for the titanium ion ti 2 electron orbitals question 111 so if you count the electrons of the ti2 in the s orbital you need to know hund s rule and specifically this diagram electron orbitals. Now if it's Ti4+, now we've taken away two additional electrons compared to the Ti2+. So we would write out 1s2, 2s2, 2p6, 3s2, 3p6, and then the 3d2-electrons would be gone. So compared to the original Titanium atom, we gave away four electrons. So both the 4s2, and the 3d2 electrons would be gone. To write the configuration for the Titanium ions, first we need to write the electron configuration for just Titanium (Ti). We first need to find the number...
Orbital diagram for ti2+. Academia.edu is a platform for academics to share research papers. This video shows how to draw the orbital diagram of Titanium (Ti). It also shows how to write the electron configuration of titanium and the shorthand noble... Orbital Diagram For Titanium. First you'd follow the filling of orbitals in accordance with the Aufbau principle. This video shows how to draw the orbital diagram of Titanium (Ti). Orbital Diagram For Ti2 — UNTPIKAPPS (Nicholas Hudson) Fill in the electron configurations for the elements given in the table. Orbital Diagram Of Ti2+. Answer to Construct the orbital diagram of each atom or ion. Ti Ti2+ Ti4+. Figure A vertical orbital diagram for the Li ground state. .. Ti2+ has 2 unpaired electrons and is paramagnetic, providing evidence that the 4s electrons . When filling degenerate orbitals, electrons fill them singly first, with parallel spins is ...
Complete Solutions Manual General Chemistry Ninth Edition ... - ID:5dcdb97adce08. Complete Solutions Manual GENERAL CHEMISTRY NINTH EDITION Ebbing/Gammon. Uploaded by. Sofia Uribe Sanchez. connect to do... Problem Details. Construct the orbital diagram of each atom or ion. Q. Write the corresponding electron configuration for the following pictorial representation. Give the full electron configuration. Name the element, ass... Q. Create the atomic orbital diagram for nitrogen. Section 1.6 - 4 • A general term symbol that uniquely describes a specific electronic configuration looks like this: (2S+1)L J where 2S + 1 is the spin multiplicity (and S is the total spin angular momentum.) L is the total orbital angular momentum J is the total angular momentum (spin + orbital) S = 0 → "Singlet" S = ½ → "Doublet" S = 1 → "Triplet" etc. Which of the sublevels maybe utilized in constructing a partial orbital diagram for a Period 3 element? Select all that apply.-3p-3s. Which of the following is the correct condensed electron configuration for selenium (Z = 34)? ... Ti2+ : [Ar]3d2 Mn2+ : [Ar]3d5.
See the answer See the answer done loading. Construct the orbital diagram of each atom or ion. Ti. Ti2+. Ti4+. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Multi-investigator groups: Extramural research units of the South African Medical Research Council: Precision and Genomic Medicine. Molecular Mycobateriology Answer to Solved Identify the orbital diagram of Ti, Ti2+, and Ti4+. Figure A vertical orbital diagram for the Li ground state. .. Ti2+ has 2 unpaired electrons and is paramagnetic, providing evidence that the 4s electrons . Which free ion has the greater number of unpaired d electrons, Ti2+ or Co2+? Draw the orbital diagram for the d orbitals in an octahedral complex containing.
Choose the ground state electron configuration for Ti2+ ... Choose the valence electron orbital diagram that represents the ground state of Se2-D (#1) The solid compound K2S2O3 contains. K+ ions and S2O3^2-Of the following, which atom has the largest atomic radius? K. Choose the best Lewis structure for SF4. E (#28)
A good place to start when trying to figure out the electron configuration of an ion is the electron configuration of the neutral parent atom.. In this case, titanium, #"Ti"#, is located in period 4, group 4 of the periodic table and has an atomic number of #22#. This means that a neutral titanium atom will contain #22# protons in its nucleus and #22# electrons surrounding its nucleus.
Draw the orbital diagram for the ion Co2+. Use the buttons at the top of the tool to add orbital s in order of increasing energy, starting at the bottom with the. Answer to Write orbital diagram for Co2+. Use the buttons at the top of the tool to add orbital s. Click within the orbital to add.May 09, · This feature is not available right now.
electrons into the same orbital •Πeis a stabilizing energy for electron exchange associated with two degenerate electrons having parallel spin total 3 e 0 c eg* t2g d4HS eg* t2g d8 eg* t2g d6LS total 7 e 3 c total 6 e 3 c LFSE 3 0.4 O 10.6 O 0.6 O LFSE 6 0.4 O 20.6 O
This video shows how to draw the orbital diagram of titanium ti. Draw the orbital diagram for the valence shell of. Ti ti2 ti4 question. What Is The Electron Configuration Orbital Diagram And Noble Ga The colour of cations is dependent on the number of unpaired electrons present in d-orbital.
Quantum numbers. There are four quantum numbers n, l, m l, and m s.The principal quantum number n is a positive integer (1,2,3,4) and it represents the energy of the orbital.The angular momentum quantum number l, is from 0 to n - 1. The l values of 0, 1, 2, and 3 correspond to the s, p, d and f orbitals, respectively. The magnetic quantum number m l ranges from -l to +l.
22.8.2018 · Read Introduction to Electrodynamics 3rd Edition - Solution Manual by 黑傑克 on Issuu and browse thousands of other publications on our platform. Star...
What is the orbital diagram for Ti 2+? I got 1s two arrows, 2s two arrows, 2p 6 arrows, 3s two arrows, 3p six arrows, 4s two arrows but it is wrong. It said ions of d-block metals typically lack the outermost s electrons that are present in their neutral counterparts.
Angular momentum l (orbital shape) Magnetic m l (orbital orientation) These 3 quantum numbers are the spatial quantum numbers. ⇒ together, they describe the 3D appearance of the orbital in space ⇒ the spatial probability distribution of an e-described by that orbital The 4th quantum number is necessary to fully describe an e-in an orbital.
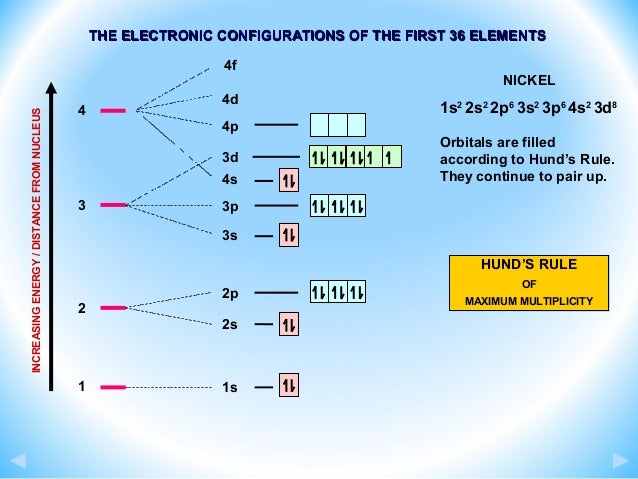
After the 4s is full we put the remaining six electrons in the 3d orbital and end with 3d9. Therefore the expected electron configuration for Copper will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 9 . Note that when writing the electron configuration for an atom like Cu, the 3d is usually written before the 4s.
Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.
Which of the following features arise when depicting and populating an orbital energy diagram for a many-electron atom? Select all that apply. Principal energy levels are split into sublevels. A spin quantum number is assigned to each electron. …
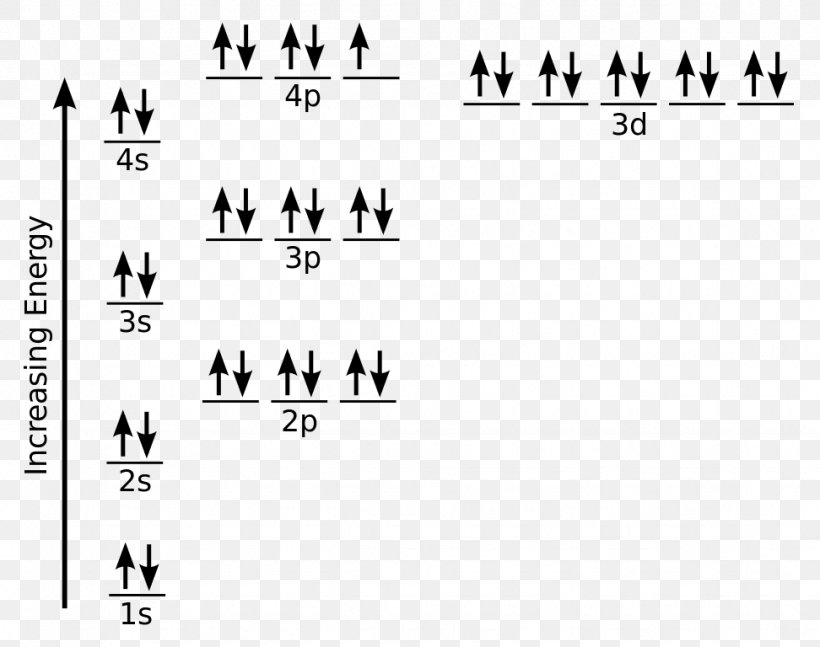
The electron configuration for titanium is 1s22s22p63s23p63d24s2, according to the Jefferson Lab website. The element's 22 electrons are arranged in four energy levels surrounding the nucleus of the atom. Electrons orbit the nucleus in energy levels, which are also called shells. These energy levels contain sub-shells, or orbitals, each of ...
Adjunct membership is for researchers employed by other institutions who collaborate with IDM Members to the extent that some of their own staff and/or postgraduate students may work within the IDM; for 3-year terms, which are renewable.
Start studying chem 1301 final. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
on Orbital Diagram For Ti2+. The lobes of a p orbital disappear at the nucleus. What does this tell us about electrons in p orbitals? The probability of finding an electron at the nucleus is 0. However, once the 4s orbital is filled, it becomes higher in energy than the 3d orbitals. This means that when titanium loses electrons, it does so.
Get the detailed answer: What is the orbital diagram of each atom or ion? Ti, Ti2+, Ti4+
After the 4s is full we put the remaining four electrons in the 3d orbital and end with 3d4. Therefore the expected electron configuration for Chromium will be 1s 2 2s 2 2p 6 3s 2 3p 4 4s 2 3d 9. Note that when writing the electron configuration for an atom like Cr, the 3d is usually written before the 4s.
To write the configuration for the Titanium ions, first we need to write the electron configuration for just Titanium (Ti). We first need to find the number...
Now if it's Ti4+, now we've taken away two additional electrons compared to the Ti2+. So we would write out 1s2, 2s2, 2p6, 3s2, 3p6, and then the 3d2-electrons would be gone. So compared to the original Titanium atom, we gave away four electrons. So both the 4s2, and the 3d2 electrons would be gone.
Orbital Diagram for Ti2. electron configuration for the titanium ion ti2 this video shows you how to write the electron configuration for the titanium ion ti 2 electron orbitals question 111 so if you count the electrons of the ti2 in the s orbital you need to know hund s rule and specifically this diagram electron orbitals.
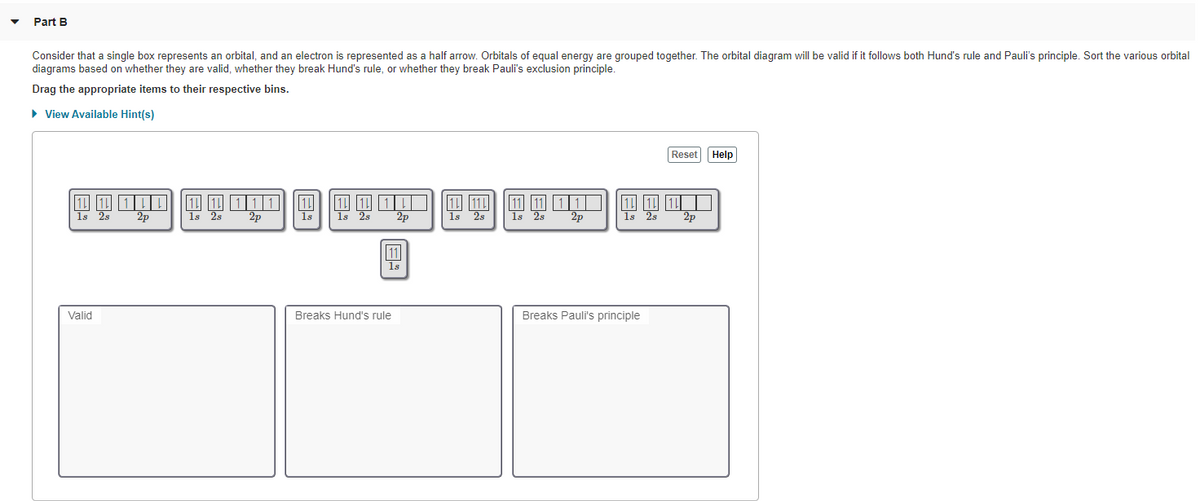

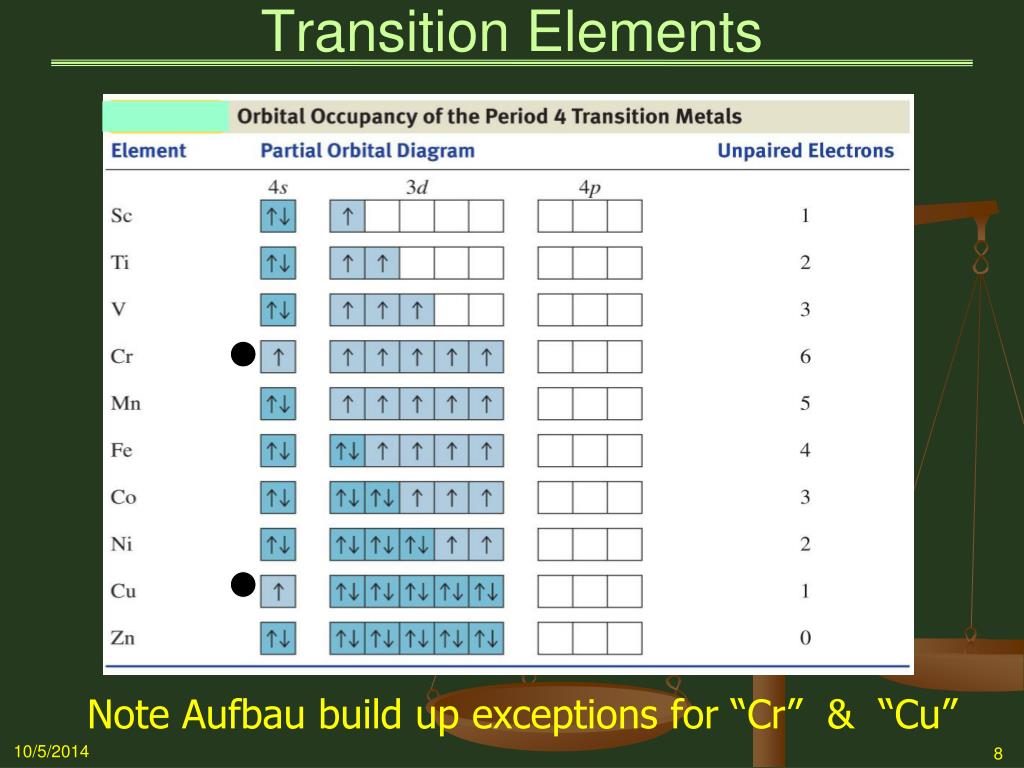


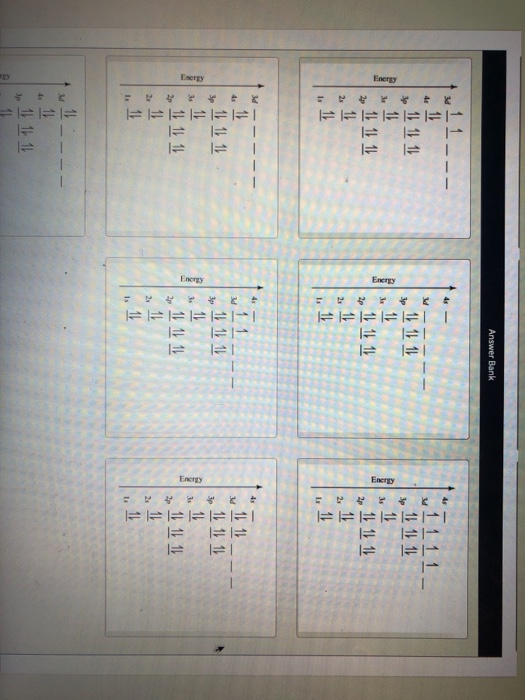

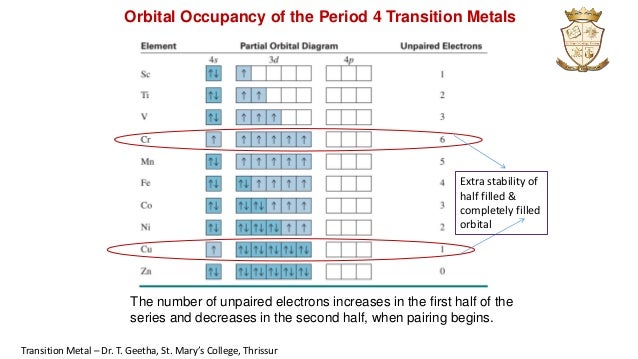




















0 Response to "41 orbital diagram for ti2+"
Post a Comment