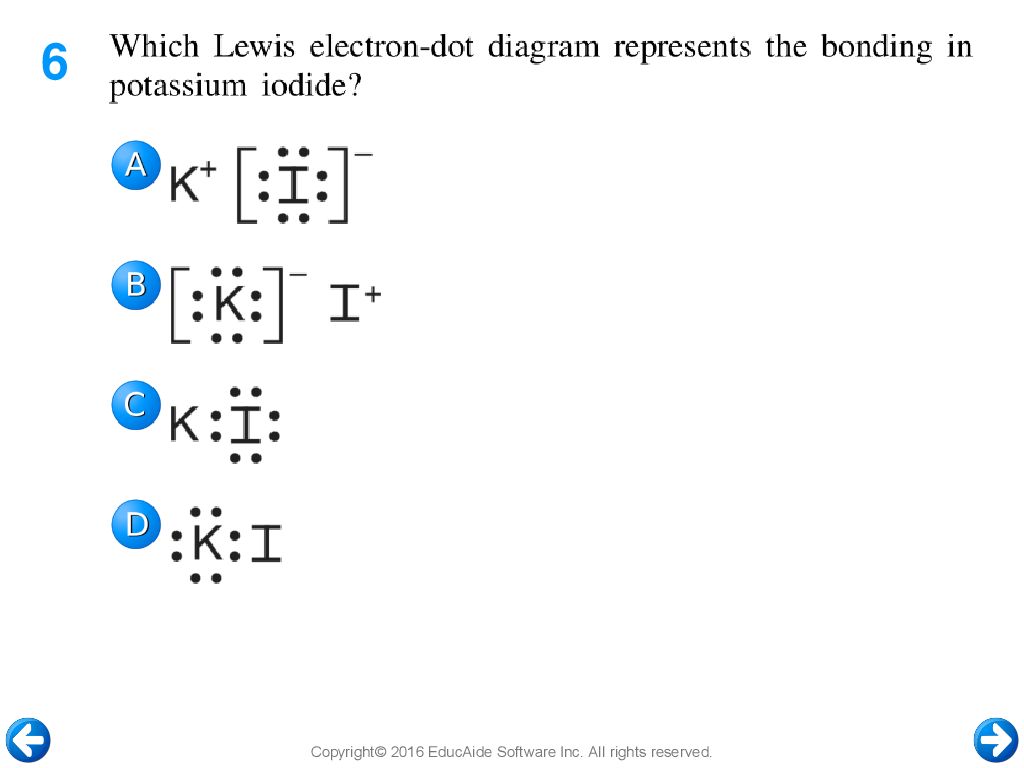
37 which lewis electron-dot diagram represents the bonding in potassium iodide
Which Lewis electron-dot diagram represents the bonding in potassium iodide? Which statement describes the energy changes that occur as bonds are broken and formed during a chemical reaction?
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
2. Which Lewis electron-dot diagram represents the bonding in potassium iodide? 3. Which equation shows conservation of mass and energy for a reaction at 101.3 kPa and 298 K? + 483.6 4. + 285.8 kJ 4. Given the formulas of two substances: These diagrams represent substances that have 1. the same molecular structure and etthe same physical properties
Which lewis electron-dot diagram represents the bonding in potassium iodide
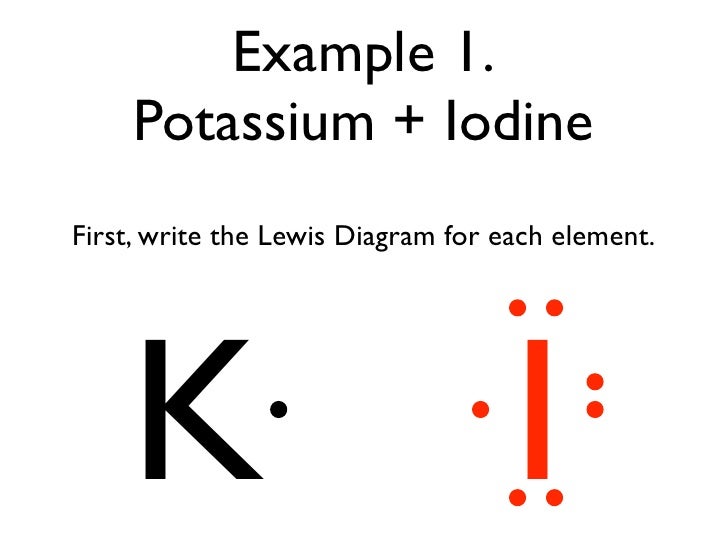
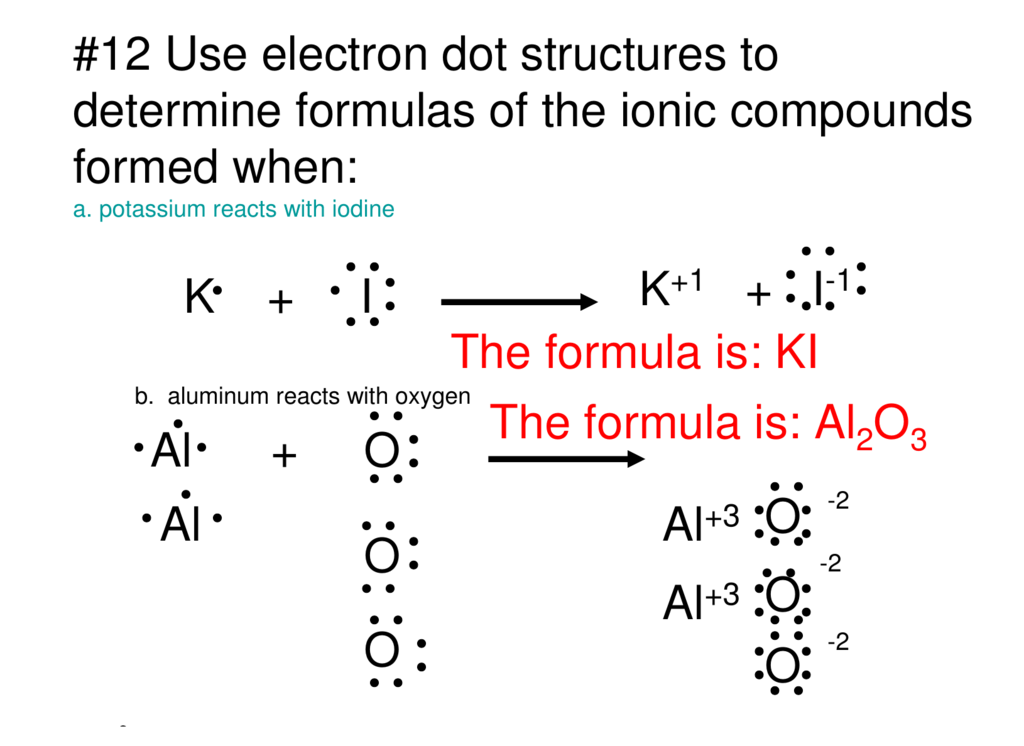
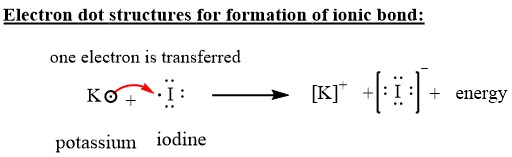
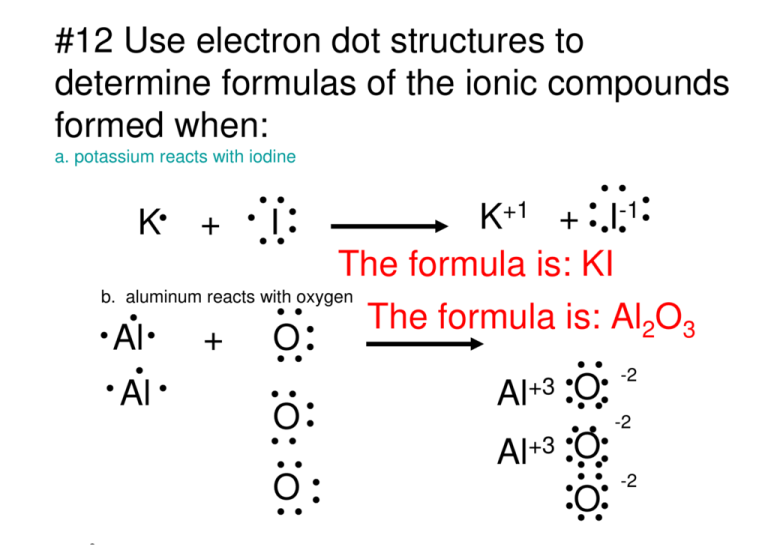
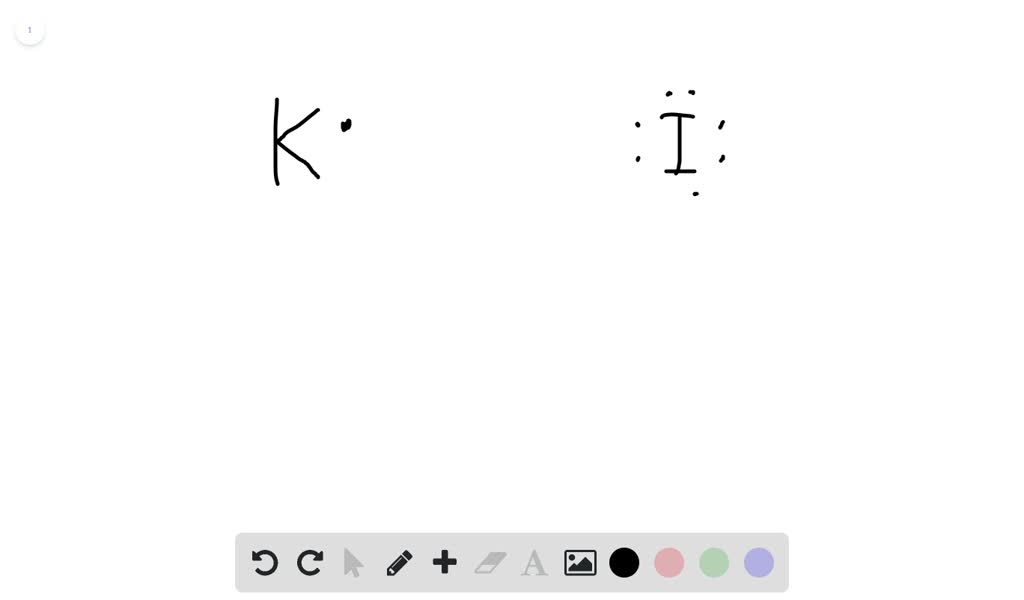
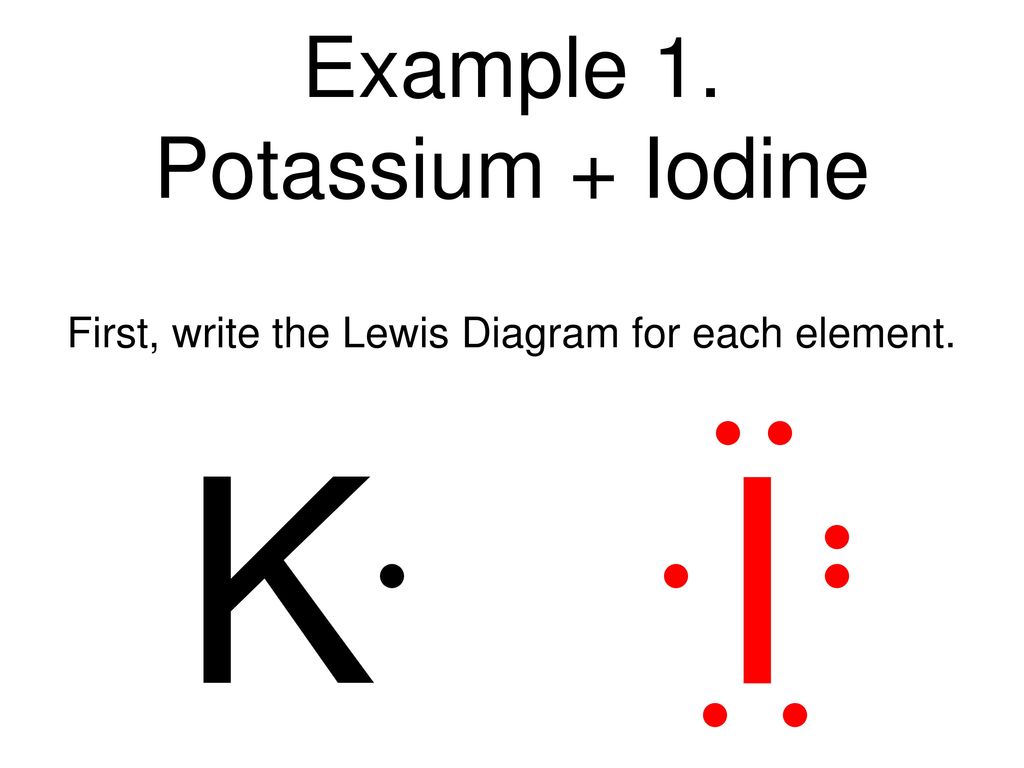
Jan 20, 2019 · The Lewis dot structure is a method of representing compounds in which the number of valence electrons on each element in the compound is shown using dots. In this case, option 1 is the correct representation for potassium iodide using dot electron structure because potassium atom transfers its one valence electron to iodine forming the iodide ion having eight valence electrons as shown.
Lewis electron dot diagram for potassium iodide ... Then we will design Lewis for ions I ions and add parentheses. 48 What diagram of Lewis electron points represents the bond in potassium iodide. The property at Lewis Dot in Cal2 is located on both sides of the Ion Ca2. Platinum atom electrons insidea molecule.
Answers: 1 to question: Which lewis electron-dot diagram represents the bonding in potassium iodide.
Which lewis electron-dot diagram represents the bonding in potassium iodide.
The Lewis dot diagram for Platinum is a diagram showing bonds & electrons of the Platinum atom within a molecule. Nobody will be able to draw you a diagram here, as this is a text-only answer ...
Potassium, a metal in Group 1, loses one electron to become a +1 ionIodine, a non-metal in Group 17, gains one electron to become a -1 ion.Together, they com...
A Lewis structure is a structural illustration of a molecule the place dots are used to show electron positions around the atoms and features or dot pairs represent covalent bonds between atoms. Lewis structures will also be made for molecules that comprise covalent bonds and for coordination compounds. …
When we write the . Lewis dot structures help predict molecular geometry. Place the remaining four electrons around the iodine atom to complete the structure. Electron Dot Structures - Helpful tools in thinking about bonding. Pictorial Electron dot structure - valence electrons are represented by dots placed around the. Solutions for Chapter ...
However,In Lewis structure or diagram,we use dot to represent valence electron.So the Lewis dot structure of Chlorine is the symbol Cl with 7 dots around it.When you draw the dots, don't just put them anywhere.Instead,imaginea square around the element's symbol.
Structure, properties, spectra, suppliers and links for: Potassium iodide, 7681-11-.
3.Base your answer to the following question on your knowledge of chemical bonding and on the Lewis electron-dot diagrams of H2S, CO2, and F2 below. Explain, in terms of structure and/or distribution of charge, why CO2 is a nonpolar molecule. 4.Base your answer to the following question on the information below and on your knowledge of chemistry.
Iodine Dot Diagram Which Lewis Dot Structure Represents Bonding In Potassium Iodide. Iodine Dot Diagram Raman Spectra A Of Anatase Tio 2 A And Iodine Doped Tio 2 B. Iodine Dot Diagram How To Draw The Lewis Dot Structure For Nai Sodium Iodide. Iodine Dot Diagram I2 Dot And Cross Diagram Wiring Diagram Review.
Which Lewis Electron-Dot Diagram Represents The Bonding In Potassium Iodide. Chemistry 0. 7380 users searched for this homework answer last month and 78 are doing it now, let's get your homework done. This Top Homework Answer is Middle School level and belongs to the Chemistry subject. This answer got 196 "Big Thanks" from other students ...
Or electron dot diagram or a lewis diagram or a lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The lewis dot diagram for neon has a pair of electrons on each side of neon symbol ne for a total of 8 electrons. As potassium iodide is a salt there is no fixed molecular ...
Potassium is K1+, and Iodine is I1-. As a result, Potassium iodide is made. It's ionic becuase Potassium has a positive charge, so it needs one more electron to have its orbitals filled.
Electron dot diagram for potassium. You draw the electron dot diagram by using the charges of the cation and anion. Et first all angles in carbon atoms are tetrahedral. It is also in the third row so that is why it moves up to the fourth energy level. It is k with one dot so. Potassium1 is a monoatomic monocation obtained from potassium.
A step-by-step explanation of how to draw the KI Lewis Dot Structure.For KI we have an ionic compound and we need to take that into account when we draw the ...
The bonds in H2O are best described as. answer choices. covalent, because valence electrons are shared. covalent, because valence electrons are transferred. ionic, because valence electrons are shared. ionic, because valence electrons are transferred. <p>covalent, because valence electrons are shared</p>. alternatives.
Lewis Dot Structure of BeI2. Lewis dot structure or electron dot structure is a representation of valence electrons on an individual atom as bond pairs or lone pairs, and the position of constituent atoms of a molecule with respect to each other. In a Lewis structure, Chemical symbols represent bonding atoms. A dot represents a valence electron
which Lewis electron-dot diagram represents the bonding in potassium iodide?(See # 6) C which formula represents a substance with the greatest degree of ionic bonding?
ko2 lewis dot structure potassium draw bonding chemical paramagnetic electron chemistry answer . ... dot potassium lewis iodide structure electron diagram bonding represents which exatin info . oxygen lewis structure dot electrons valence dots system atom facilitates represent uses wps .
Each O is surrounded by four dots and two sticks or lines, representing another 4 electrons in the O2 double bond. So each O is surrounded by 8 total valence electrons, giving it an octet and making it stable. The two letter O's in the O2 Lewis structure represent the nuclei (centers) of the oxygen atoms.
Lewis structures (also known as Lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of atoms within a molecule. Why is potassium oxide K2O? Potassium oxide is an ionic compound formed by combining potassium and oxygen.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...
Quizlet A model of an atom in which each dot represents a valence electron. CLICK THE Calcium, potassium, and sodium are classified under this Oxidation Number. Positive or Lewis Structure. Explanations. 31 Which electron shell contains the valence electrons of a radium atom in the ground state? 35 Which Lewis electron dot diagram represents a.
Lewis Dot Structures can be produced by following a sequence of steps. Let's produce a Lewis Dot Structure for: NH 4 + (the ammonium ion). Step 1: Count valence electrons: N = 5 4 x H = 4 x 1 = 4 "+" = -1 Total = 5+4-1= 8 electrons = 4 bonds and lone pairs. Step 2:!Arrange the atoms (identify a central atom, if possible).
The lewis dot diagram for platinum is a diagram showing bonds electrons of the platinum atom within a molecule. That means it has 7 valence electrons so we have 7. Iodine is in group 7 of the periodic table. Iodine is in group 17 on the periodic table meaning that iodine needs only a single electron to become chemically stable.
Electron dot diagram for potassium. The oxygen atom has a charge of 2. To form an ionic bond an atom will either lose or gain electrons. Potassium is a group one element with a charge of 1 and oxygen with a charge of 2 a lewis dot diagram should contain two potassium atoms and one oxygen atoms to show that they form an ionic bond.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The bifluoride on heating yields potassium fluoride: Platinum or heat resistant plastic containers are often used for these operations.
![Expert Verified] Which Lewis dot structure represents bonding ...](https://us-static.z-dn.net/files/dcb/eb0cb0a538e5db52ff692f01fa6f54ea.png)
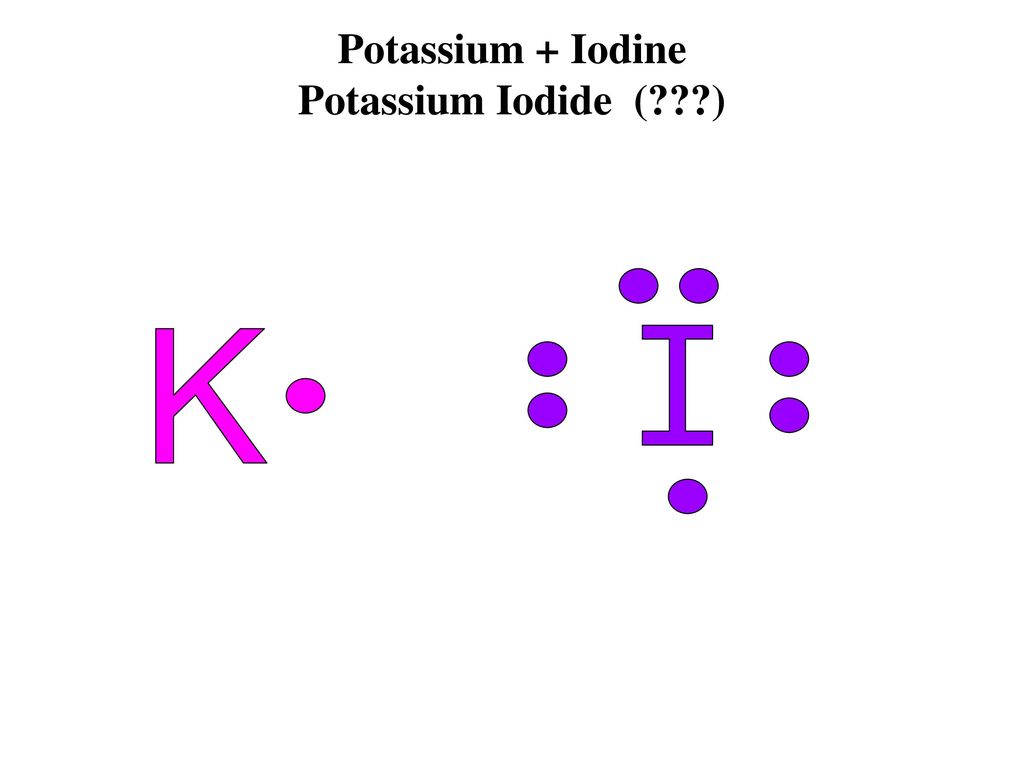






![Expert Answer] Write the electron dot structure for potassium ...](https://us-static.z-dn.net/files/dae/42ea31befae64e0549ebc6aa19bd29da.png)










0 Response to "37 which lewis electron-dot diagram represents the bonding in potassium iodide"
Post a Comment