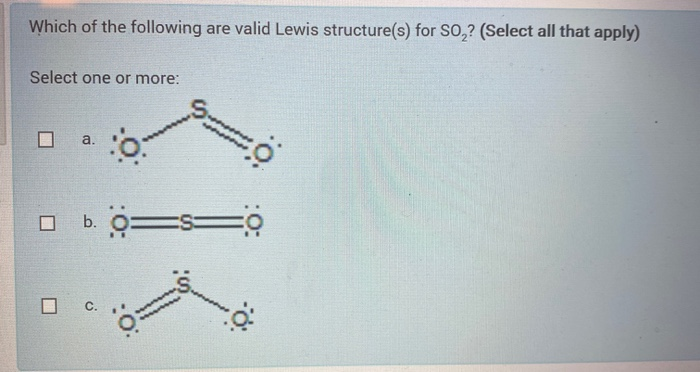
37 lewis diagram for so2
SeO2 Lewis Structure, Geometry, Hybridization, and Polarity. The chemical formula of selenium dioxide is SeO2. It is a unidimensional polymer chain having alternating selenium and oxygen atoms. This chemical compound is of great importance because of its corrosive nature for metals only when in contact with water. To create the Lewis structure of SO2 you need to arrange the eight valence electrons on the Sulphur. So there is a need for a double bond. Gain Dish Soap Again. In SO2 lewis structure there are two double bonds between sulfur atom and oxygen atoms. In this editor I will have to write it as Ö-S -Ö.
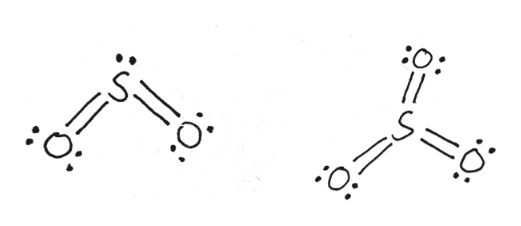
In SO2 lewis structure, there are two double bonds between sulfur atom and oxygen atoms. Sulfur dioxide lewis structure is drawn step by step using VESPR ...
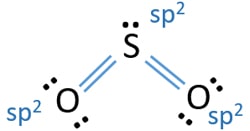
Lewis diagram for so2
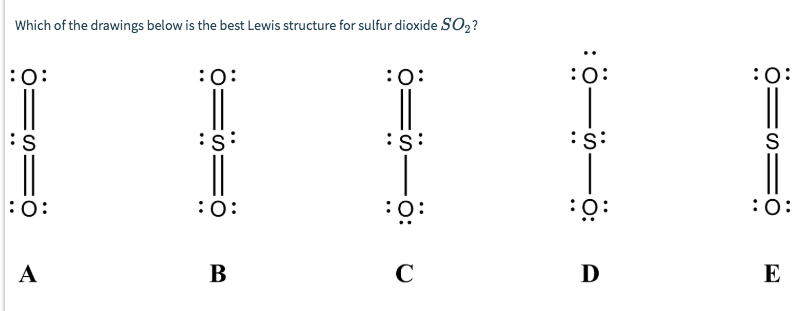
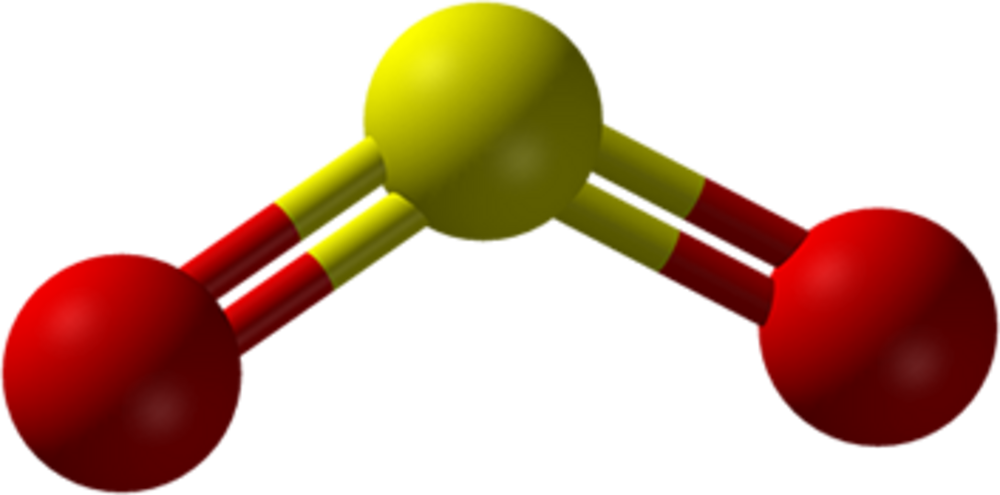
Lewis Structure of Carbon Dioxide ( C O 2). In ( C O 2), oxygen belongs to group 16 of the Periodic Table, and carbon belongs to group 14 of the Periodic Table. Hence, oxygen has 6 valence electrons, and carbon has 4 valence electrons. Step 1- In ( C O 2) we, have C = 4 × 1 = 4 valence electrons. By analyzing the Lewis structure of SO2, we can see that the SO2 is asymmetrical because it contains a region with different sharing. The molecular geometry of SO2 has a bent shape which means the top has less electronegativity, and the bottom placed atoms of Oxygen have more of it. So, the conclusion is, SO2 is a Polar molecule. Lewis Structure. Popularly known as electron dot structure or Lewis dot structure is a representation of the bonds which are formed in between the elements of a molecule with the help of a diagram. You must also read out the article written on the lewis structure, geometry of SO2.
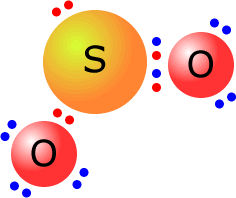
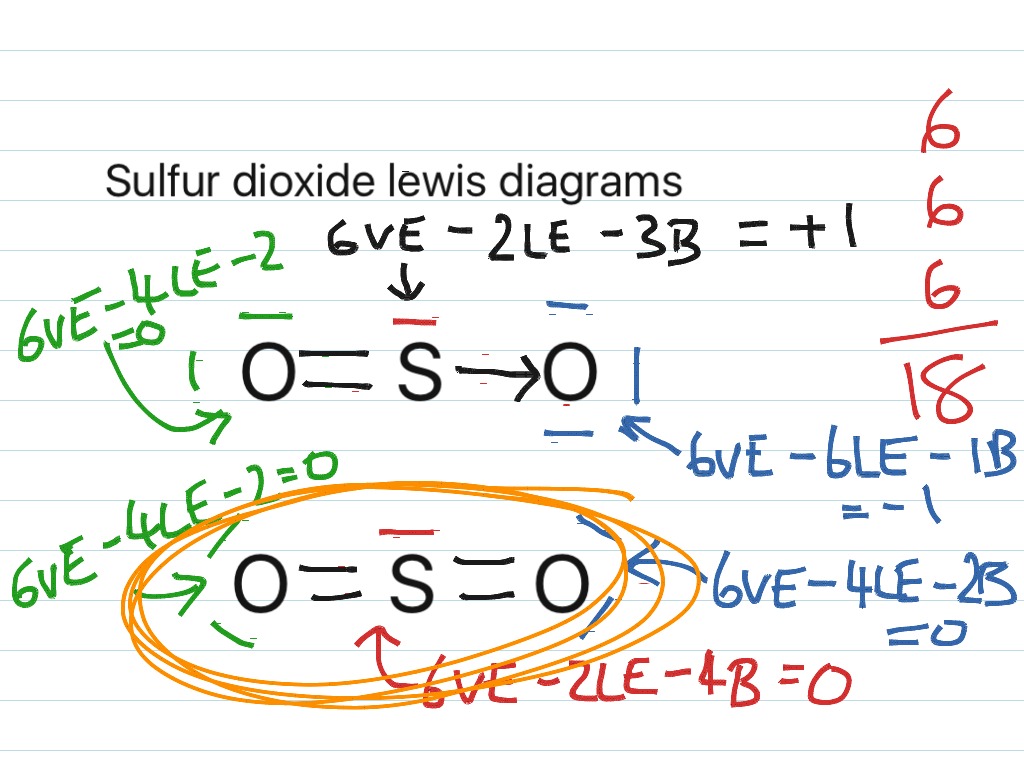
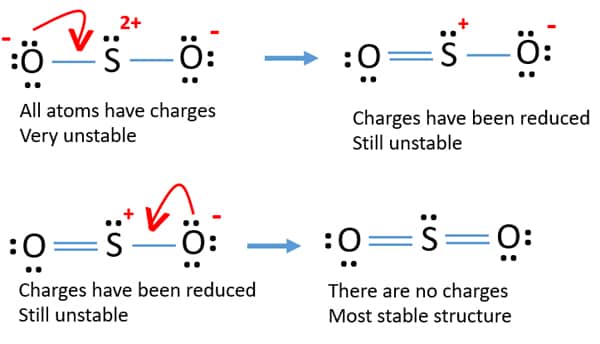
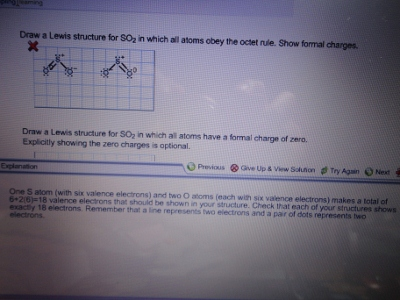
Lewis diagram for so2. Draw a Lewis structure for SO2 in which all atoms have a formal charge of zero. Explicitly showing the zero charges is optional. Do not consider ringed - 20921592 Lewis Structure of Sulphur dioxide. Sulfur dioxide is a chemical compound that has SO2 as its chemical formula. It's a toxic chemical that has the scent of a burning matchstick. SO2 is commonly generated by volcanic activity and is created by burning fossil fuels tainted with sulfur compounds. It is a gas that is thick, colorless, and poisonous. SO2 Lewis Structure Before directly jumping into the lewis structure of SO2, let's have a quick discussion regarding the importance of lewis structure and the steps to draw it. Lewis structure is the distribution of the electrons around the atoms of a compound. SeO2 Lewis Structure, Molecular Geometry, Shape and Bond Angle. The chemical formula SeO2 represents the chemical compound Selenium Dioxide. It is a colorless solid and one of the most available forms Selenium. Selenium is a non-metallic element that finds use in semiconductors, glass-making, and supplements.
ClO2- Lewis Structure, Geometry, Hybridization, and Polarity. ClO2 is the molecular formula of Chlorine dioxide that is commonly used to treat potable water. It is far better than chlorine because it has higher solubility in water and does not hydrolyze unlike chlorine, and resides as dissolved gas. In ionic form chlorine dioxide is known as ... SO2 Lewis structure. To create the Lewis structure of SO2, you need to arrange the eight valence electrons on the Sulphur. To design the best Lewis structure, you also need to calculate the formal charge of every atom too. You know that both the Sulphur and Oxygen has six valence electrons each. Silicon Dioxide (SiO2) Lewis Structure. The Lewis structure of SiO2 is identical to the Lewis structure of CO2. The only difference is that instead of carbon, silicon is used. One silicon atom is at the middle, with two oxygen atoms bound to it in a double bond. There are no lone pairs on the central atom of the SiO2 Lewis dot structure, The Lewis dot structure of SO2 or sulfur dioxide has a central atom of sulfur that violates the octet rule. It causes a repulsion of electron pairs to form the 120-degree angleBy analyzing the Lewis structure of SO2 we can see that the SO2 is asymmetrical because it contains a region with different sharing.
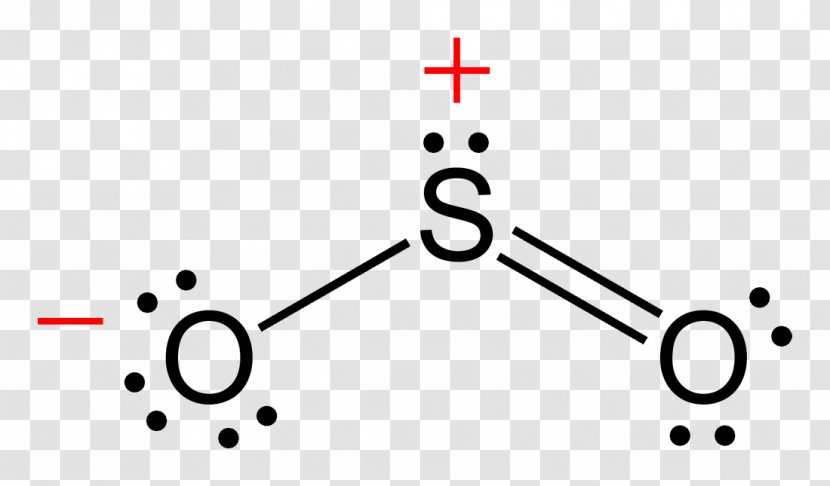
SO2 - A Detailed Discussion. Sulfur dioxide (SO 2) is a sulfur and oxygen-based gaseous air pollutant. Moreover, SO 2 is a poisonous, heavy, and colorless gas and emits the odor of burned matches. When sulfur-containing fuels like coal, oil, or diesel undergo combustion, SO 2 is produced. Besides, one can also create it as a by-product of ... With SO2 Lewis Structure, the central atom is the central sulfur atom because of the higher valence of the sulfur atom than the oxygen atom. The SO2 Lewis Structure provides the best explanation of how the sulfuric acid (1) transformed into such after dissecting the bonds of Sulfur and Oxygen. This can be a hazard to one's health, but this is ... For calculating the formal charge of SO2, we have to unlock its Lewis structure. Therefore, the first step of calculating formal charge is drawing the Lewis structure. SO2 has the following Lewis structure: how to calculate the formal charge Numbers 1,2,3,4 represent the Oxygen Atom Index. How To Draw Lewis Dot Structure Of So2 2/8 Download made for a single atom, a covalent compound, or a polyatomic ion. Using the Periodic Table to Draw Lewis Dot Structures CH3COOH lewis structure, molecular geometry, polarity Now we will draw the lewis structure of acetic acid step by step with all possible
SO2 Lewis Framework. Before quickly delving into the lewis structure of SO2, allow's have a short conversation concerning the usefulness of the lewis framework and the actions to draw it. Lewis structure is the positioning of the electrons around the atoms of a substance. This framework benefits us to learn about the sort of bonds and the ...
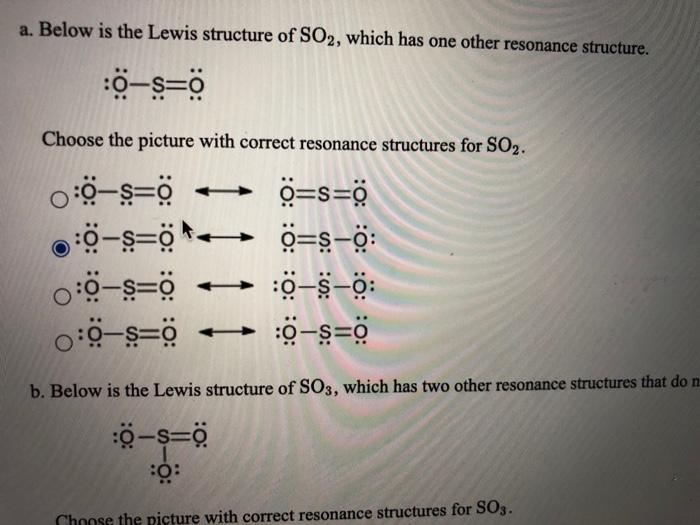
Lewis structure of SO3. The sulfur trioxide is a tetra atomic chemical molecule where both the sulfur and three oxygen molecules bond with an equal number of valence electrons. The diagram is drawn showing dots of valence electrons around the symbol of both sulfur and oxygen atoms with lines predicting bond formation.
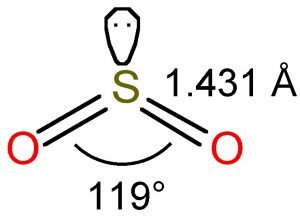
The bond angle in SO2 = 120 degrees. For more detailed information regarding geometry, hybridization, and lewis structure of SO2, you should also refer to the article on the lewis structure of SO2. Polar and Non-Polar Compounds. We all should understand that when two atoms form a bond, they basically share electrons from each other.
Lewis Structure. Popularly known as electron dot structure or Lewis dot structure is a representation of the bonds which are formed in between the elements of a molecule with the help of a diagram. You must also read out the article written on the lewis structure, geometry of SO2.
By analyzing the Lewis structure of SO2, we can see that the SO2 is asymmetrical because it contains a region with different sharing. The molecular geometry of SO2 has a bent shape which means the top has less electronegativity, and the bottom placed atoms of Oxygen have more of it. So, the conclusion is, SO2 is a Polar molecule.
Lewis Structure of Carbon Dioxide ( C O 2). In ( C O 2), oxygen belongs to group 16 of the Periodic Table, and carbon belongs to group 14 of the Periodic Table. Hence, oxygen has 6 valence electrons, and carbon has 4 valence electrons. Step 1- In ( C O 2) we, have C = 4 × 1 = 4 valence electrons.







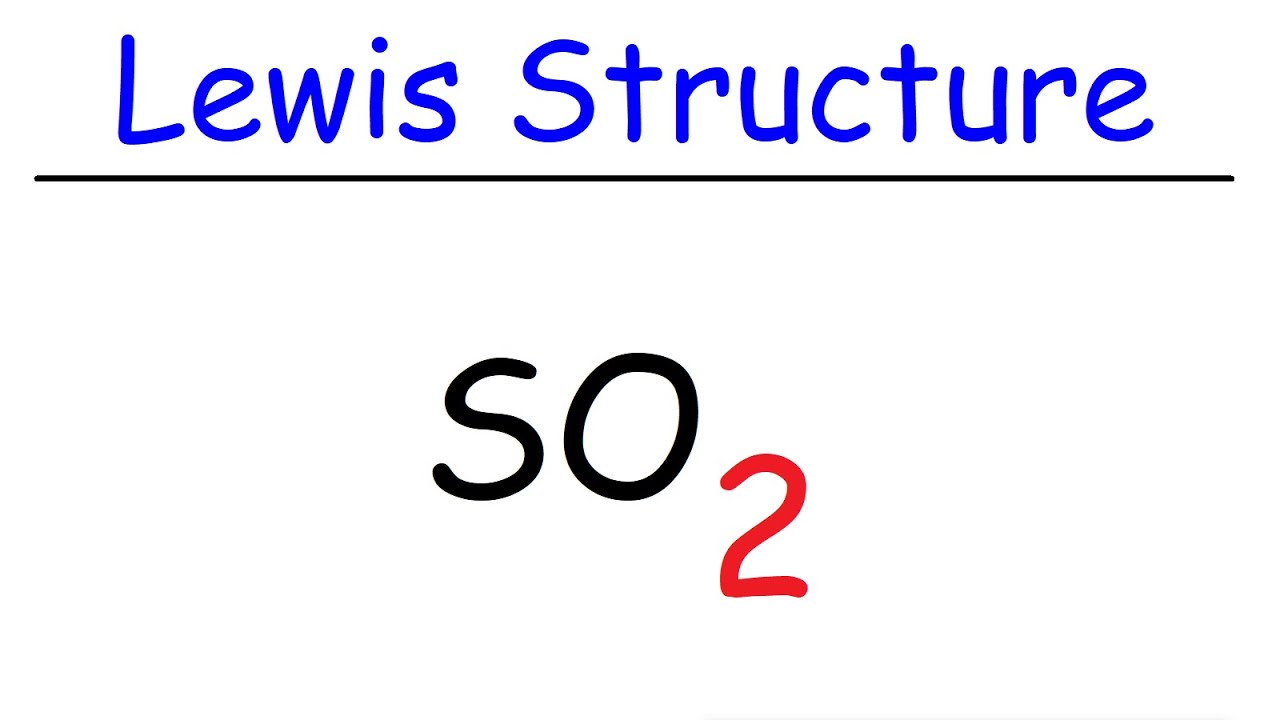















0 Response to "37 lewis diagram for so2"
Post a Comment