37 lewis diagram for nh3
Alternatively a dot method can be used to draw the nh 3 lewis structure. First draw the lewis dot structure. Nh3 Lewis Stru...
Lewis dot diagram of nh3 the lewis structure of ammonia, nh3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons. Please feel to comment if you have any questions. Drawing the lewis structure for nh 3 (ammmonia) ammonia (nh 3) is a...
Which Lewis electron dot diagram correctly represents NH3? Explanation: Figure C is the correct Lewis dot structure of Ammonia. How many valence electrons does NH have? There are 8 valence electrons available for the Lewis structure for NH3.

Lewis diagram for nh3
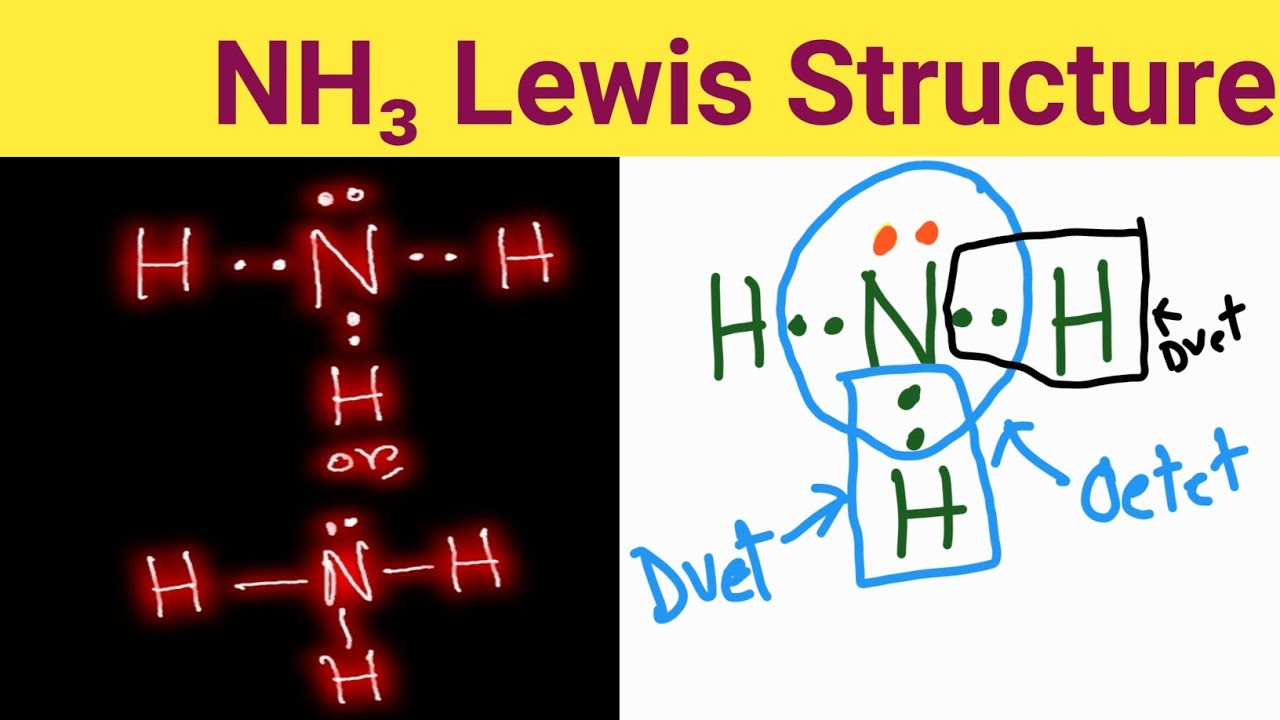
Drawing the Lewis Structure for NH 3 ( Ammmonia) Ammonia (NH 3) is a commonly tested Lewis structure due to it's widespread use in agriculture as a fertilizer. The NH3 Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the NH3 molecule.
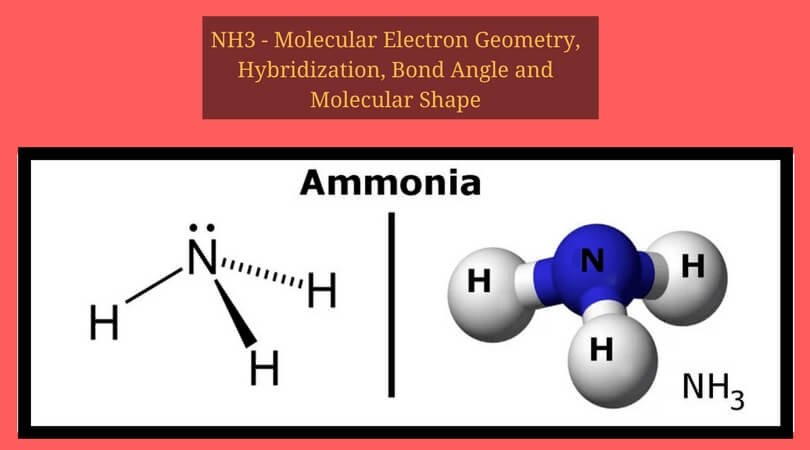
Molecular Geometry of NH3. Trigonal Pyramidal. And to understand the Lewis structure, we first need to find out the valence electrons in this molecule. Now that we know the valence electrons for the molecule, we can predict its Lewis structure. Hydrogen atoms never take the central position, so we...
Drawing the Lewis Structure for NH3 (Ammmonia). Ammonia (NH3) is a commonly tested Lewis structure due to it's widespread use in agriculture as a fertilizer. It also is a good example of a molecule with a trigonal prymidal molecular geometry.
Lewis diagram for nh3.
Drawing the lewis structure for nh 3. Lewis structure of nh 3. Electron Dot Diagram Nh3 There are 8 valence elec...
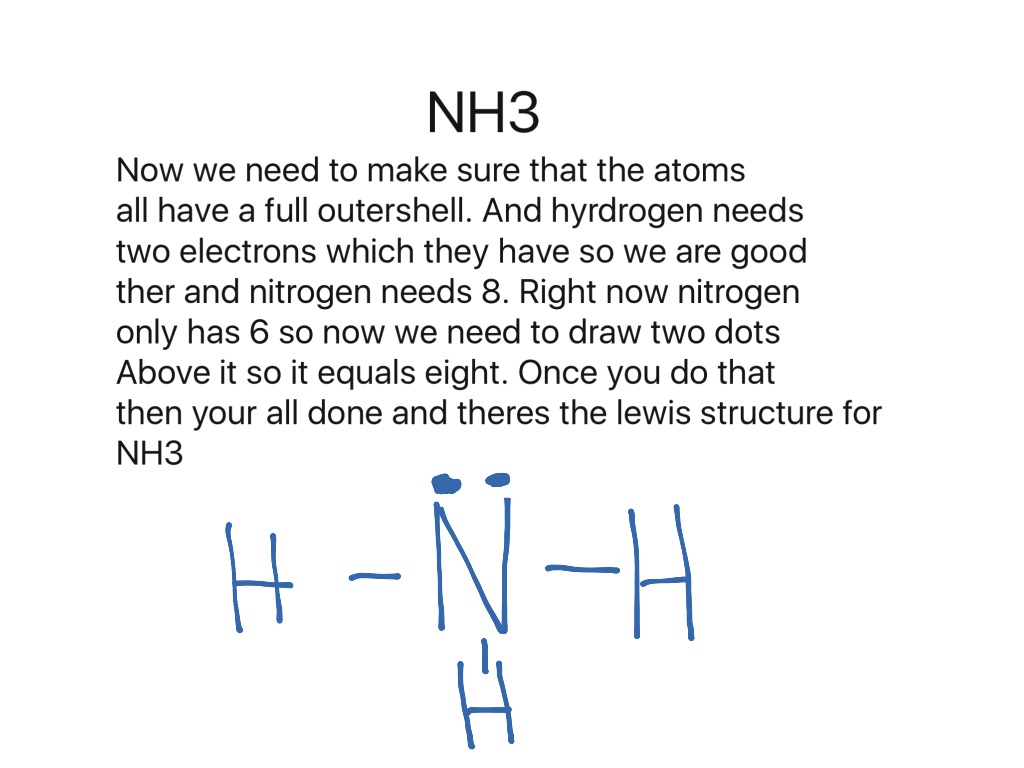
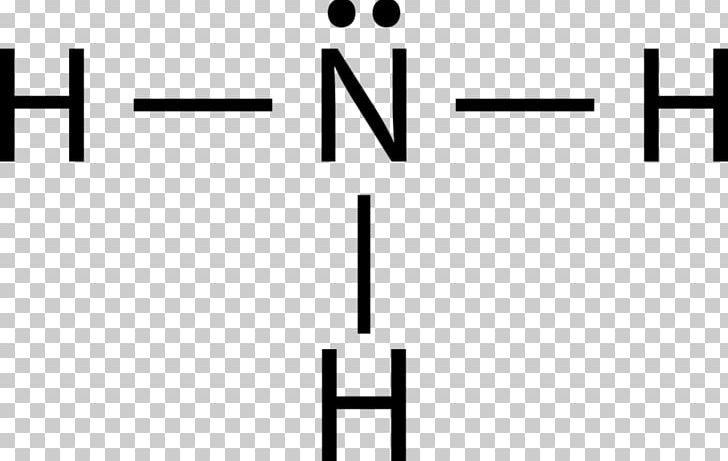
In the Lewis structure for NH 3 there are a total of 8 valence electrons. Three pairs will be used in the chemical bonds between the N and H and one pair of That's the Lewis structure for NH3. You'll see it drawn a lot as a structural formula. That's going to look like this right here, where these electrons are...
7 hours ago Lewis dot diagram for nh3. In the nh 3 lewis structure and all structures hydrogen goes on the outside. 2 hours ago How is the lewis dot diagram for nh3 determined quora e thing you know right off the bat is that nitrogen is in the middle hydrogen only has one valence electron so it will...
Lewis used simple diagrams (now called Lewis diagrams) to keep track of how many electrons were present in the outermost, or valence, shell of a given atom. Draw Lewis diagrams for an atom of each of the following elements: Li, N, F, Na. Solution. NH3, N2H4, HN3.
A step-by-step explanation of how to draw the NH3 Lewis Dot Structure (Ammonia).For the NH3 structure use the periodic table to find the total number of...
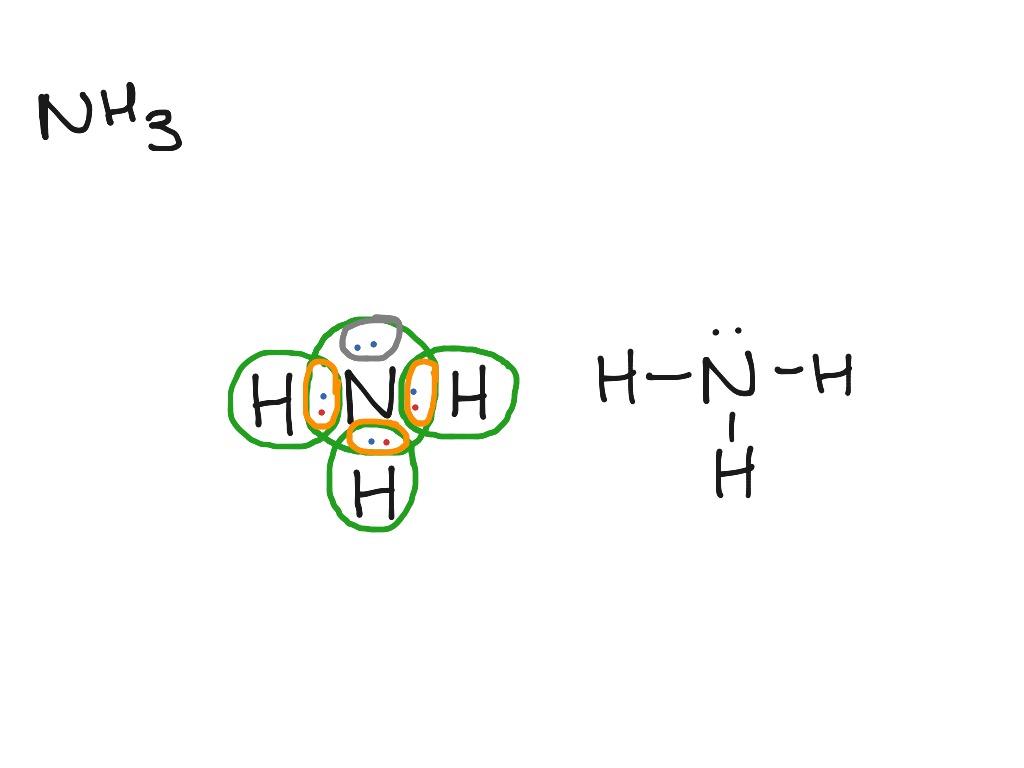
Lewis Structures or electron dot diagrams for atoms, ions, ionic compounds and covalent compounds tutorial with worked examples for chemistry students. Each of the 3 hydrogen atoms in NH3 will share its electron with the central nitrogen atom to form a bonding pair of electrons (covalent bond) so...
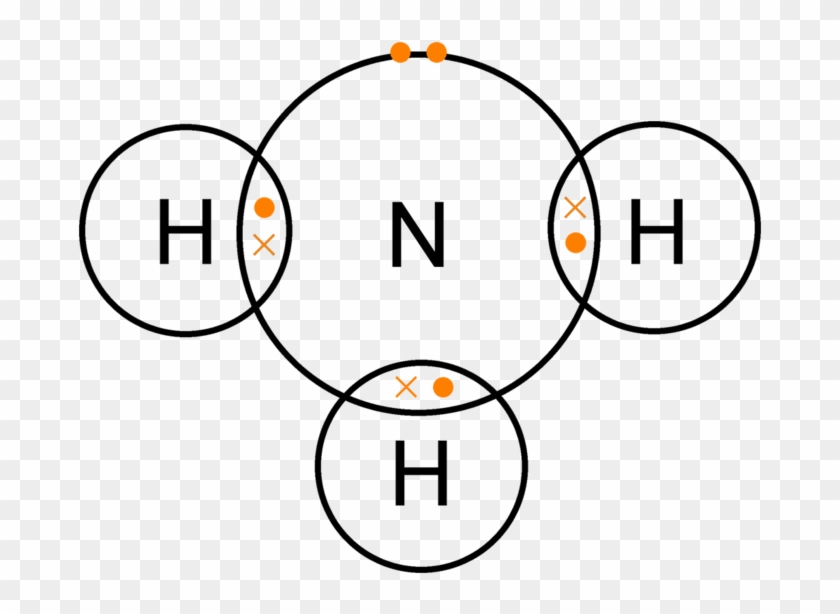
(Lewis diagram of ammonia) simplified 'dot and cross' electronic diagram for the covalently bonded ammonia molecule. The ammonia molecule is held together by the strong N-H nitrogen-hydrogen single covalent bonds by sharing electrons. Note that the inner shell of nitrogen's electrons are not...
Nh3 Lewis Diagram! study focus room education degrees, courses structure, learning courses. 1 week ago The NH3 Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the NH3 molecule.
Lewis dot diagrams show all the valence electrons (outermost energy levels) making up the bond, also indicating how the pairing occurs as in this covalent bond.
Nitrogen begins with 5 electrons and the hydrogens each start with 1 electron. Drawing the lewis structure for nh 3. Draw A...
/thumb\/e\/ef\/Draw-Lewis-Dot-Structures-Step-3-Version-3.jpg\/v4-728px-Draw-Lewis-Dot-Structures-Step-3-Version-3.jpg","smallWidth":460,"smallHeight":306,"bigWidth":728,"bigHeight":485,"licensing":"<div class...
To understand the NH3 Lewis structure, we need to understand the structure of Ammonia (NH3). In NH3, Nitrogen has 5 electrons in the valence shell, so it needs to combine with 3 hydrogen atoms to fulfil the octet rule and form a Importance of Lewis Structure. Rules for drawing Lewis dot structures.
Lewis diagram NH3. by. Jayne Ferguson 8 years ago. Viewed after searching for: Lewis electron dot diagram for CH4.
Drawing the lewis structure for nh 3 ammmonia ammonia nh 3 is a commonly tested lewis structure due to it s widespread use in agriculture as a fertilizer. 022 lewis diagrams and vsepr modelsin this video paul andersen explains how you can use lewis diagrams and vsepr models to make...
For drawing Lewis diagrams the one that you should be familiar with is the spectroscopic notation. For example the electron configuration of chlorine in The Lewis diagrams for hydrogen and chlorine are: Notice the single unpaired electron (highlighted in blue) on each atom. This does not mean this...
Lewis structure of nh 3. Calculate the total valence electrons in the molecule. Solved Clearly Draw The Lewis Electron Dot...
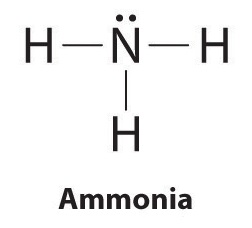
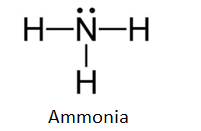
The n atom then has two dots on the unconnected side. The lewis dot structure for nh3 starts with an n atom connected on three sides...
Ammonia nh 3 is a commonly tested lewis structure due to its widespread use in agriculture as a fertilizerit also is a good example of a mo...
The Lewis structure of NH3 is made in such a manner that the scarcity of one valence electron in Look for the total number of bonds forming: Three single covalent bonds between each oxygen and hydrogen atom. Draw the lewis diagram as below: Geometrical Structure of the Ammonia (NH3).
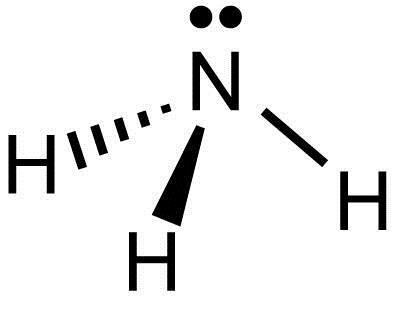
Drawing NH3 Lewis Structure is very easy to by using the following method. Here in this post, we described step by step method Step-3: Lewis dot Structure for NH3 generated from step-1 and step-2. In the NH3 Lewis structure diagram, the nitrogen atom can be the center atom of the molecule.
Details: The Lewis structure for NH3 is.The Lewis dot structure for NH3 starts with an N atom connected on three sides with a dash, each to an H atom. Details: lewis nh3 structure dot diagram bond covalent ammonia molecular angle bonds shape geometry hybridization bonding mishkanet...
Lewis structure of NH3 can be drawn by starting from valence electrons of nitrogen and hydrogen atoms in several steps. Each step of drawing the In the lewis structure of NH3, there are three N-H bonds and one lone pair on nitrogen atom. There are no lone pairs on hydrogen atoms which cannot...
NH3 Molecular Geometry Of sp3 Hybridization. The sp3 hybridized orbitals repel each other and they are directed towards four corners of a regular Lewis Structure Of NH3. Ammonia, otherwise called Nitrogen Trihydride, is a form of a colourless gas. On the periodic table, nitrogen is considered in...
Is Hi a covalent hydride? What is the Lewis diagram for NF3? In the lewis structure of Nitrogen trifluoride (NF3), there are three N-F bonds and Therefore the molecules are far less polar and the boiling point of NF3 is what might be expected from the molecular mass. In NH3 the electronegative...








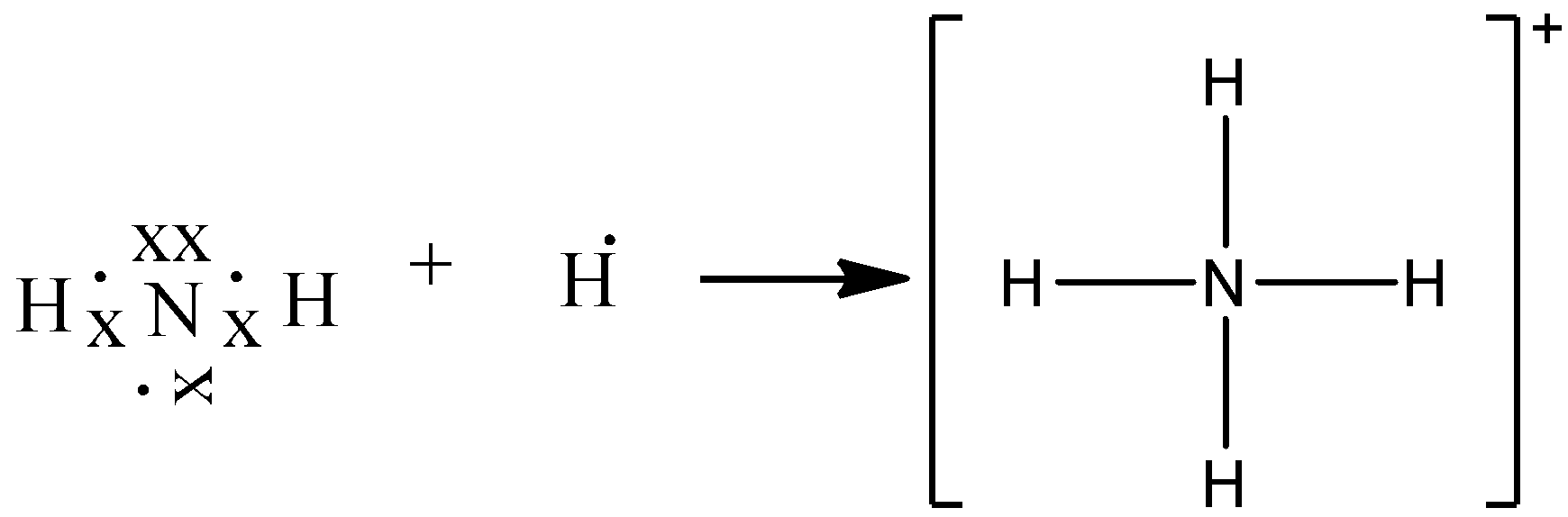
![Solved] Two students made the Lewis dot diagrams of NH3. The ...](https://us-static.z-dn.net/files/da4/66daa2f82875e459e2b8f9f507019d20.png)









0 Response to "37 lewis diagram for nh3"
Post a Comment