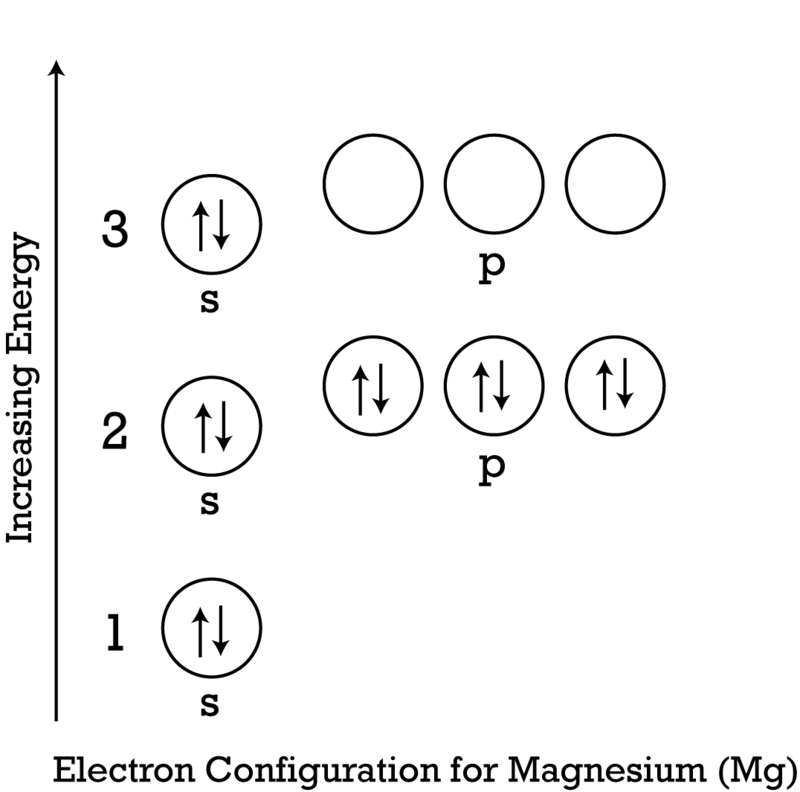
41 orbital diagram of magnesium
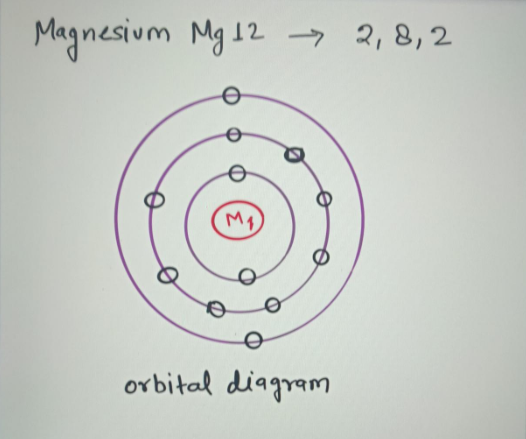
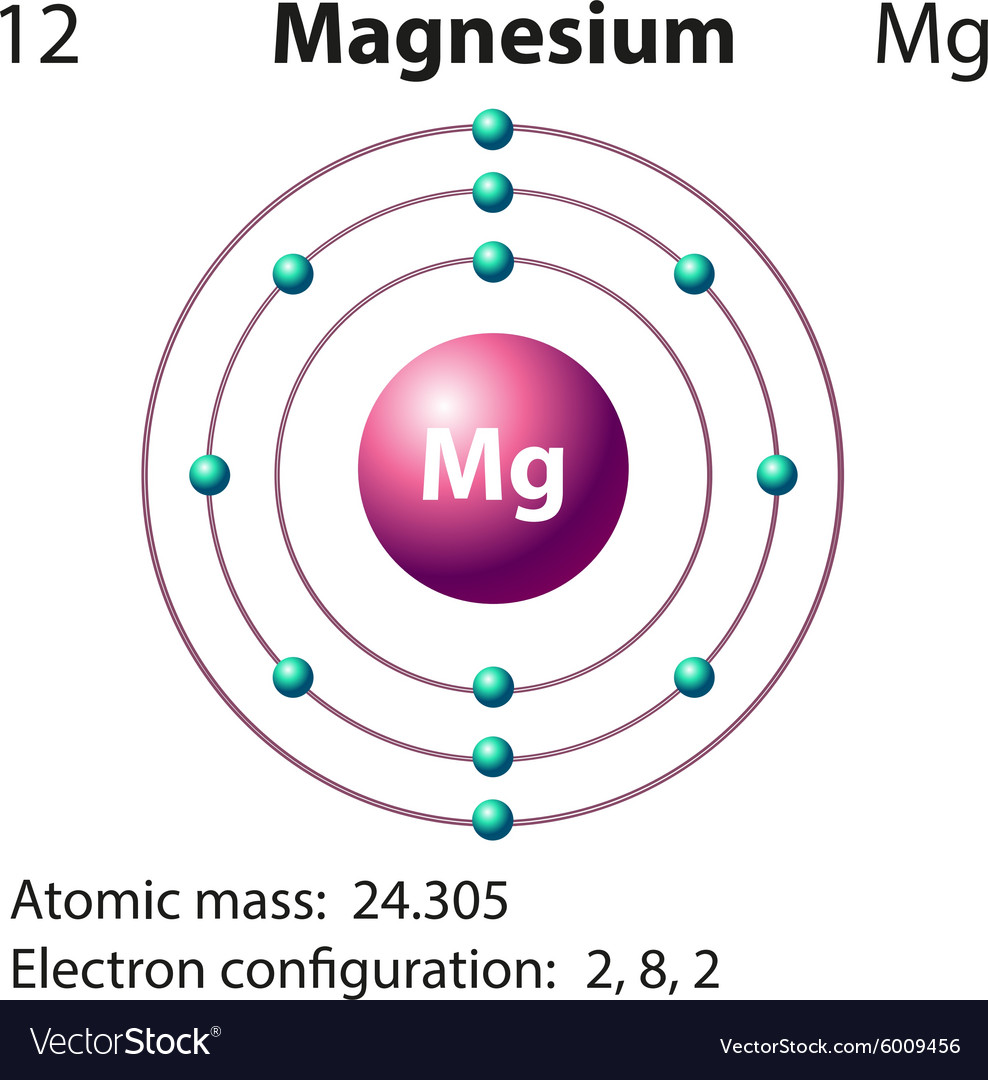
The Bohr Model of Magnesium(Mg) has a nucleus that contains 12 neutrons and 12 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Magnesium contains 2 electrons that also called valence electrons. Write the full orbital diagram for magnesium. Orbital Diagram: The subatomic particle that occupies most of the volume of an atom is known as the electron. The region of space wherein electrons ...
That is, magnesium is a cation element. Magnesium donates the electron of the last shell to form bonds and turns into magnesium ions. Mg - 2e - → Mg +2. The electron configuration of magnesium ions is 1s 2 2s 2 2p 6. The electron configuration of magnesium-ion shows that magnesium ions have only two shells and that shell has eight electrons.
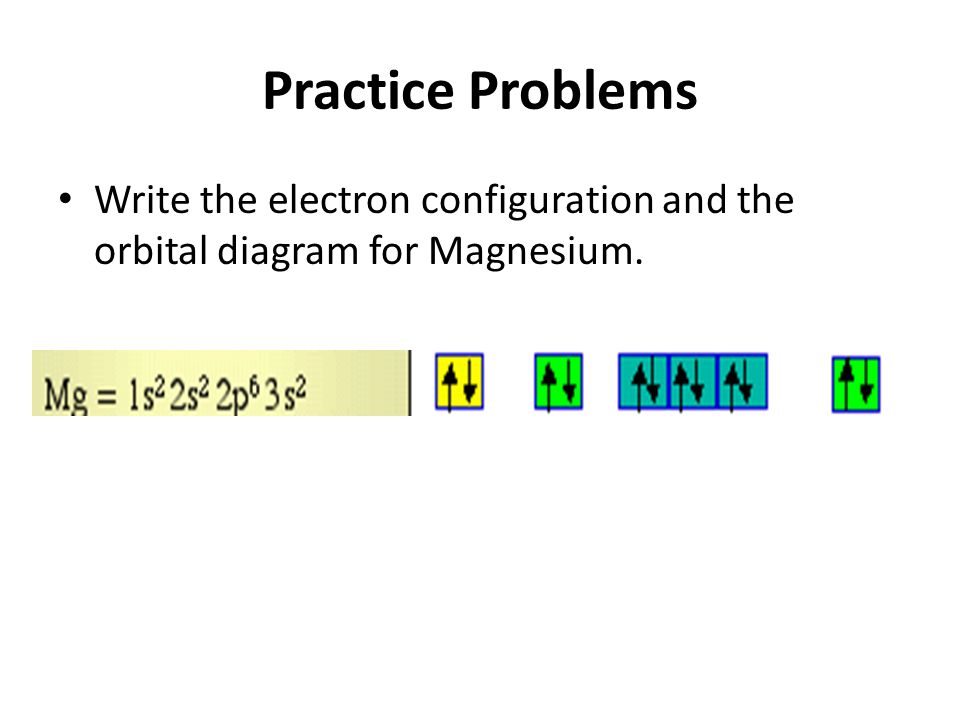
Orbital diagram of magnesium
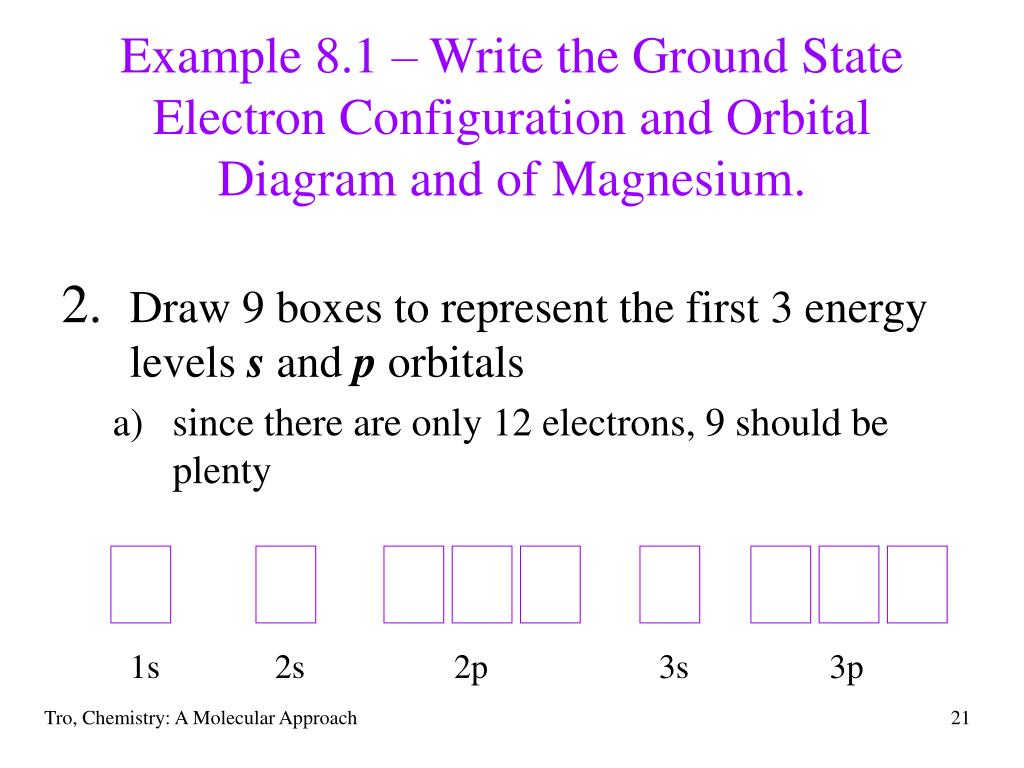
Orbital energy diagrams are provided to guide you learn about the atomic orbital. A chemical equation shows the chemical formulas of substances that are reacting and the substances that are produced. Depict the electron configuration for magnesium ing an orbital box diagram and noble gas notation. So this question is asking us to construct an orbital diagram to show the electron configuration for a neutral magnesium atom. So we're looking at magnesium ...1 answer · Top answer: We’re being asked to construct the orbital diagram for Mg. For that, we first need to determine the electron configuration of Mg.Recall that for a ... The lewis dot structure for magnesium is an mg with 2 dots which stand for its two valence electrons. What is an bond electron transfer how electron dot diagram for s awesome sulfur atom flow block orbital diagram for magnesium awesome lewis electron dot diagrams. Magnesium reacts with sulfur to produce sulfide a in.
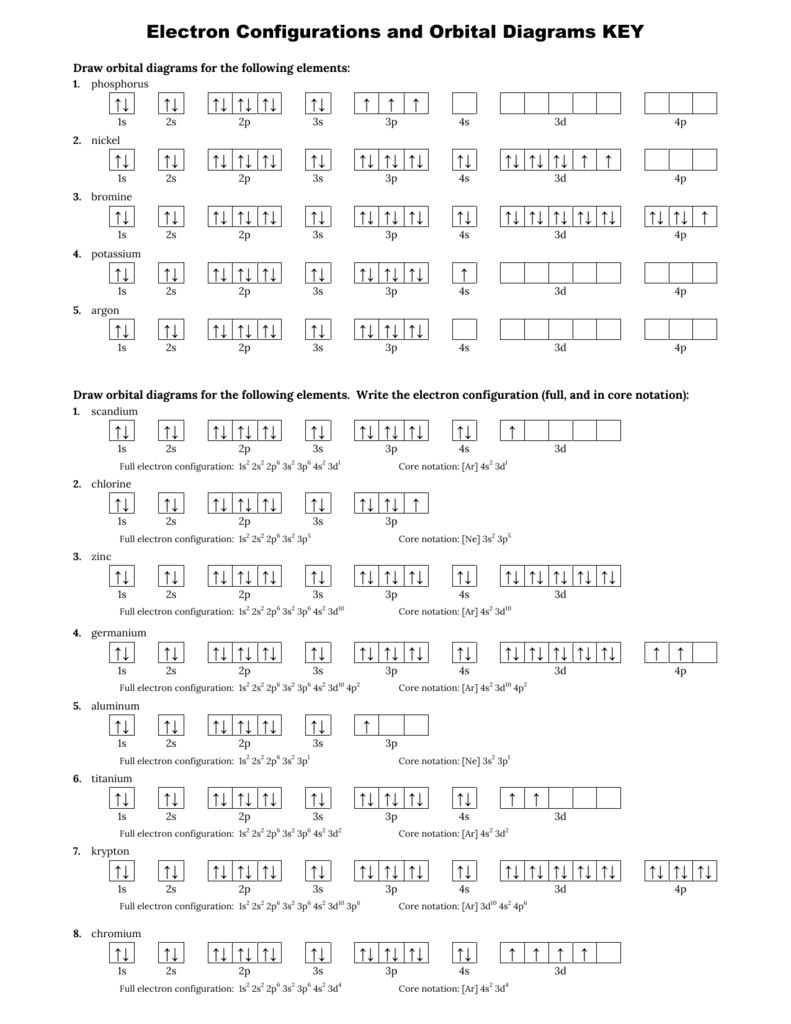
Orbital diagram of magnesium. Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ... FREE Answer to [6] (a) Write an orbital diagram for the ground state of Magnesium (Mg) and Phosphorous (P). (Marks = 1) [6] (b) Which of the following ...1 answer · 0 votes: 1) 2) Phosphorous is Paramagnetic because it contain unpaired electrons in orbital and magnesium is diamagnetic because all electrons are paired. Please ... Orbital energy diagrams are provided to guide you learn about the atomic orbital. The following orbital energy diagrams are available in printable quality to show you the mathematical function of electrons in magnesium, boron trifluoride, helium, and chlorine. Explore the diagrams in the following images, simply click to save and print! This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
Give the Orbital Diagram of the Following: Magnesium Chloride . CISCE ICSE Class 9. Question Papers 10. Textbook Solutions 19257. Important Solutions 6. Question Bank Solutions 14520. Concept Notes & Videos 431. Syllabus. Advertisement Remove all ads. Give the Orbital Diagram of the Following: Magnesium Chloride - Chemistry ... What is the orbital diagram and electron configuration of magnesium? We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s22s22p63s2. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around ... Answer (1 of 3): Orbital Diagrams Many times it is necessary to see all the quantum numbers in an electron configuration, this the purpose of the orbital diagram. In addition to listing the principle quantum number, n, and the subshell, ℓℓ, the orbital diagram shows all the different orientation... Hydrogen (H) Electron Configuration with Full Orbital Diagram. Hydrogen electron configuration is 1s 1. Hydrogen is a s-block element. This article gives an idea about the electron configuration of hydrogen, period and groups, valency and valence electrons of hydrogen, bond formation, compound formation, application of different principles.
Aug 6, 2021 — Order of Electron Arrangement. n, The diagram below shows us how electrons are arranged around each shell or orbital and how many each shell can ... a) The atomic number of magnesium is 12, and its electronic configuration is as follows, 1s 2 2s 2 2p 6 3s 2.Therefore, the orbital diagram for magnesium can be drawn as, The orbital diagram for MAgnesium is. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down ... In writing the electron configuration for Calcium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Calcium go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two ...
The electronic configuration for magnesium is 1s^2 2s^2 2p^6 3s^2. The outermost electron is one in the 3s orbital, which means that n = 3. It also means that l = 0, since s is basically shorthand for the orbital shape of l = 0. m_l can range from -l to +l, but since l = 0, then m_l must also be 0.
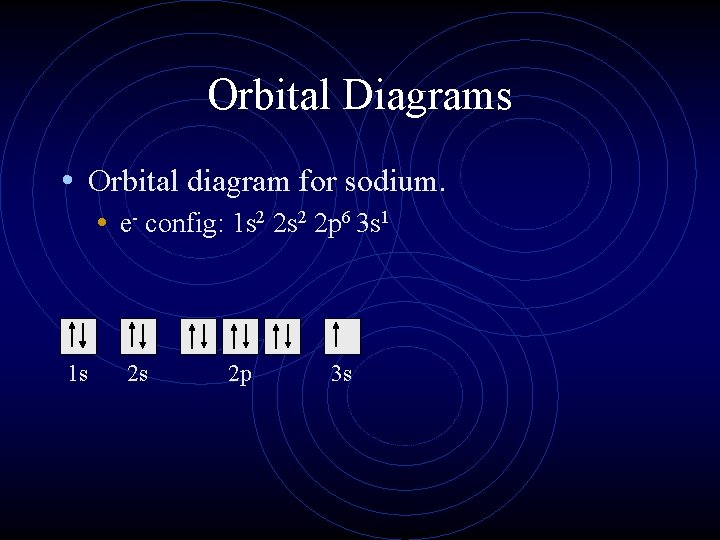
layer (row #), s = orbital type , power of 2 = the 2 electrons in the 1s orbital **Move the Helium box next to Hydrogen (above Beryllium.) See the periodic table below. Looking at the periodic table, we need to count each box going from Hydrogen (#1) to Magnesium (#12) , including Magnesium. H and He boxes: These boxes are in the 1. st row, so 1.
Answer: This just shows energy levels so let's take this a step further. Atomic Electron Configurations And I'm not having any luck but if you go to this site, you should be about to see what the 1s, 2s, 2px, 2py, 2pz, and 3s orbitals look like together. Jmol orbital structures If not, see what...

12 Mg Magnesium Electron Shell Structure Schoolmykids Electron Configuration Magnesium Element Chemistry
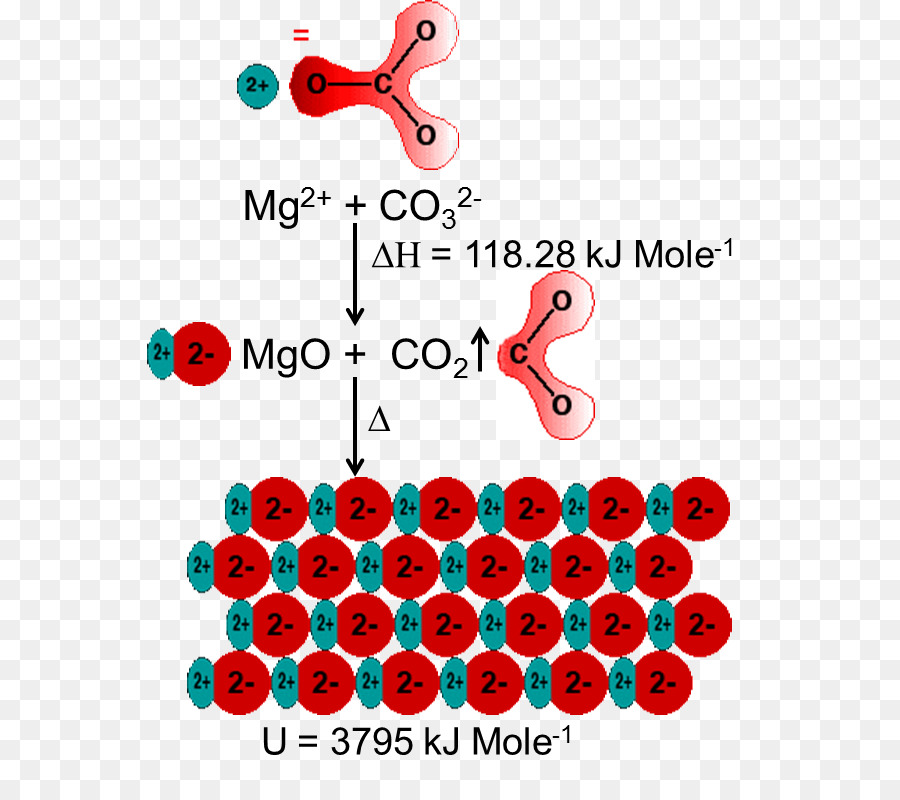
The magnesium oxide is a non-metal ionic compound that is formed because of electrostatic forces of attraction where no rules of covalent molecules, such as molecular geometry, hybridization, molecular orbital (MO) diagram, and polarity are followed.
Since 1s can only hold two electrons the next 2 electrons for magnesium go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s 2 2s 2 2p 6 3s 2.
Exam 4 Review: Ch.8-9. Electromagnetic radiation with a wavelength of 745 nm appears as red light to the human eye. The energy of one photon of this light is ________ J. Calculate the wavelength (in nm) of the blue light emitted by a mercury lamp with a frequency of 6.19 × 10^14 Hz. Nice work!
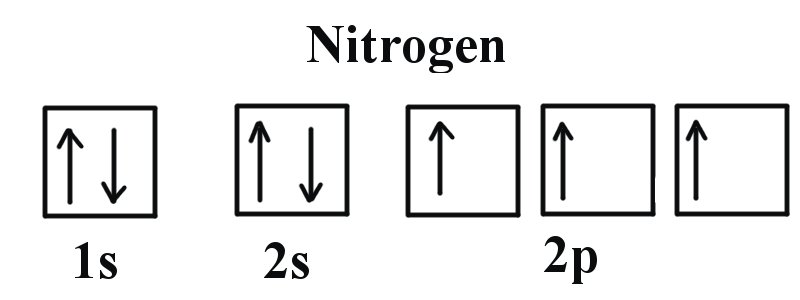
Nitrogen molecule (N 3) - . N (7) 1s 2 2s 2 2p 3 When we construct the MO diagram for nitrogen , we will only draw the valence 2s and 2p orbitals. In nitrogen MO , there is a slight change in MO diagram when compared to the MO diagrams of O 2, F 2 molecules.Here,t he σ 2p orbital is higher in energy than the π 2p orbital. This is because, the σ 2p orbital in oxygen / fluorine is more ...
The electrons fill into the orbitals following the order 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, with each s orbital holding ...1 answer · 2 votes: This just shows energy levels so let's take this a step further. Atomic Electron Configurations ...
At http://ecampus.oregonstate.edu/chemistry, you can earn college credit for online Chemistry and virtual labs. With no onsite visits required for 100 level...
Click here👆to get an answer to your question ️ Draw an electron dot diagram to show the formation of each of the following compounds:Magnesium Chloride. [H = 1, C = 6, Mg = 12, Cl = 17] .
How does an orbital diagram to show the electron configuration for a neutral magnesium atom look. The p orbital can hold up to six electrons. In order to write the mg electron configuration we first need to know the number of electrons for the mg. Electron configurations and orbital diagrams key draw orbital diagrams for the following elements ...

9 Give Orbital Diagram Of The Following Begin Array Ll Text A Magnesium Chloride B Nitrogen Text C Methane Text D Hydrogen Chloride End Array
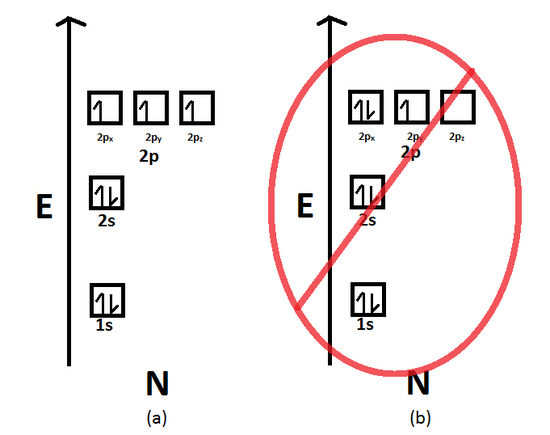
Fill the s orbital in the second energy level (the 2s orbital) with the second two electrons. What is the basis for exceptions to the Aufbau diagram? Exceptions to the Aufbau principle are based on the fact that a few atoms are more stable when their electrons fill or half-fill an electron shell or subshell.
Magnesium (Mg) has an atomic mass of 12. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.
Give orbital diagram of the following: magnesium chloride, Medium. Open in App.
Magnesium Electron Configuration (Mg) with Orbital Diagram. Magnesium Electron Configuration: Mg is a chemical element that has the symbol Mg. The atomic number of Magnesium is 12. It is a grey shiny solid that bears a close physical resemblance to five other elements of the second column and group 2 (alkaline earth metals) of the periodic table.
The lewis dot structure for magnesium is an mg with 2 dots which stand for its two valence electrons. What is an bond electron transfer how electron dot diagram for s awesome sulfur atom flow block orbital diagram for magnesium awesome lewis electron dot diagrams. Magnesium reacts with sulfur to produce sulfide a in.
So this question is asking us to construct an orbital diagram to show the electron configuration for a neutral magnesium atom. So we're looking at magnesium ...1 answer · Top answer: We’re being asked to construct the orbital diagram for Mg. For that, we first need to determine the electron configuration of Mg.Recall that for a ...

Write The Electron Configuration For Magnesium A 12 And Potassium A 19 Draw Their Orbital Brainly Ph
Orbital energy diagrams are provided to guide you learn about the atomic orbital. A chemical equation shows the chemical formulas of substances that are reacting and the substances that are produced. Depict the electron configuration for magnesium ing an orbital box diagram and noble gas notation.
Solved Construct An Orbital Diagram To Show The Electron Configuration For A Neutral Magnesium Atom Mg Mg Drag The Appropriate Labels To Their Res Course Hero

6 A Write An Orbital Diagram For The Ground State Of Magnesium Mg And Phosphorous P Marks 1 6 B Which Of The Following Elements Would Be Expected To Be Paramagnetic






















0 Response to "41 orbital diagram of magnesium"
Post a Comment