40 how to draw an enthalpy diagram
This video explains the parts of a pressure-enthalpy diagram for a single-component system and discusses how enthalpy depends on pressure for water. If these diagrams are a breeze for you, or you're just looking for help with another concept, check out other chemical engineering tutorials in the Learn ChemE Engineering Screencast series . 205.24°F (some pressure-enthalpy diagrams show the critical temperature as 204.81°F) and the critical pressure is 722.39 psia.The critical point for R-410A occurs at 161.83°F and 714.5 psia. Notice that a reference point for enthalpy is estab-lished on the graph for R-22 and on the graph for R-410A. On both graphs, the enthalpy is considered
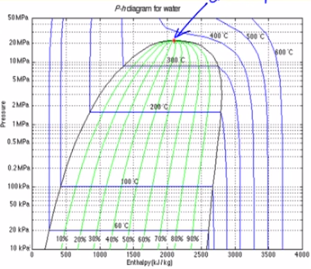
How to Read a Pressure-Enthalpy Diagram In chemical engineering, it is often necessary to know how properties like pressure, enthalpy, volume, and entropy relate to each other at a certain temperature. A Pressure-Enthalpy Diagram provides this information but can be cryptic to decipher. What you need to know: Liquid/vapor dome region
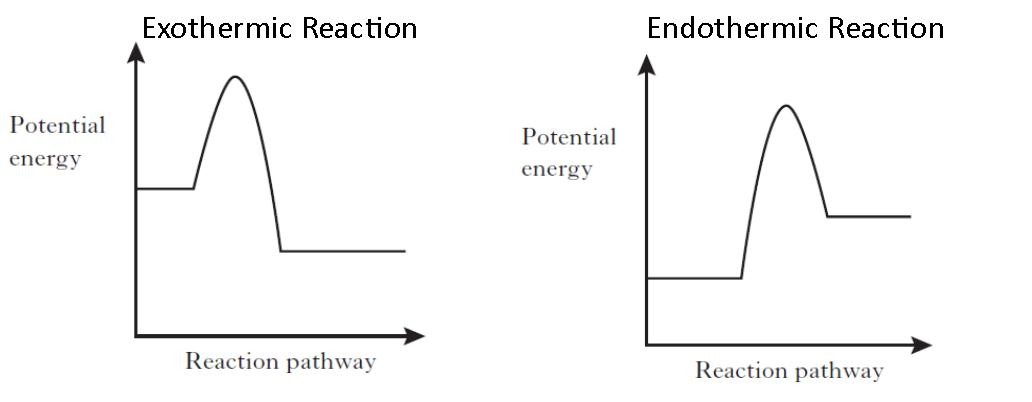
How to draw an enthalpy diagram
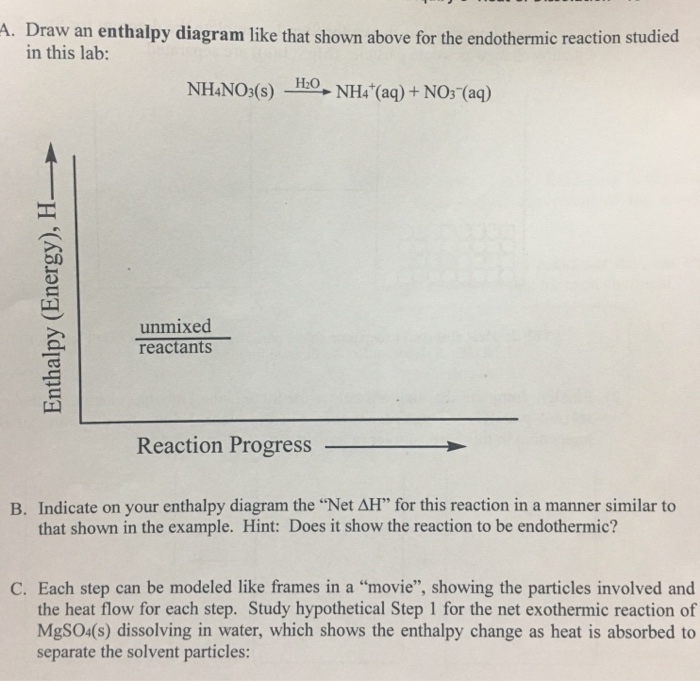
Find step-by-step Chemistry solutions and your answer to the following textbook question: Draw an enthalpy diagram for a general exothermic reaction; label the axis, reactants, products, and $\Delta \mathrm { H }$ with its sign.. a) Draw an enthalpy level diagram for a neutralization reaction. i) Indicate on your diagram the enthalpy change of the reaction and deduce its sign. ii) Compare the relative stabilities and strengths of the bonds of the reactants and products. [4] iii) Define the term standard enthalpy change of a reaction. [1] 13. (In diagrams of this sort, we often miss off the standard symbol just to avoid clutter.) Then fit the other information you have onto the same diagram to make a Hess's Law cycle, writing the known enthalpy changes over the arrows for each of the other changes.
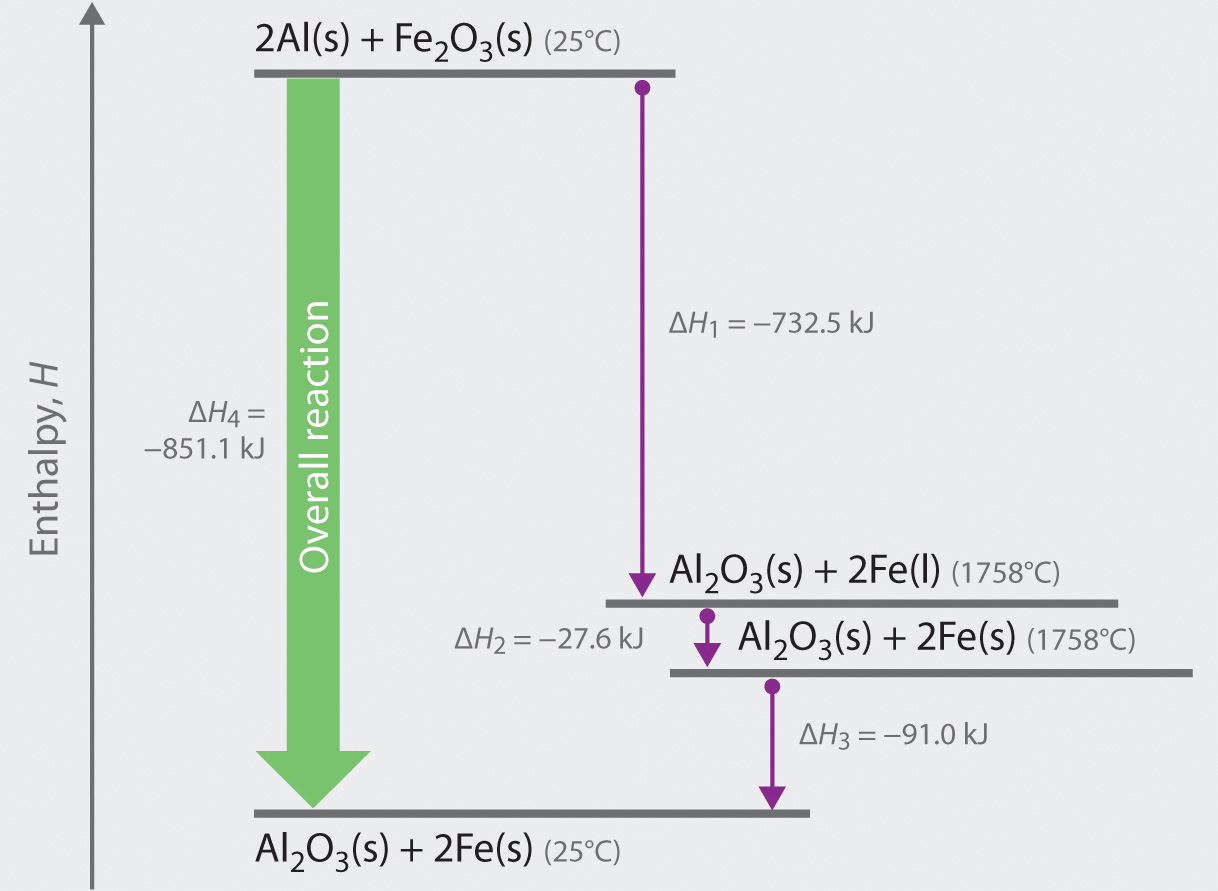
How to draw an enthalpy diagram. How to draw enthalpy diagram s from a chemical reaction an d a dH value.Table of Contents:00:14 - Learning Objectives A ch an ge in enthalpy is the difference between the enthalpy of the products an d the enthalpy of react an ts. How to draw enthalpy diagrams from a chemical reaction and a dH value.Table of Contents:00:14 - Learning Objectives The enthalpy level diagram can now be constructed. In this case, the red dotted line is obtained by subtracting the small blue dotted line from the longer blue dotted line. That is, the enthalpy of 4 - 2 = -463 - (-124) = -339 kJ. Therefore the standard enthalpy of formation, ΔH f, of phosphorus(III) chloride = -339 kJ In this video we want to learn how to draw the energy cycle given enthalpy changes of formation of compounds and calculate the enthalpy change of a reaction ...
I want to draw a rankine cycle T-S and P-V plots for my research article. I want to know any good software for plotting it. Many of the diagrams are present in Organic Rankine cycle papers. Hi everyone and welcome back to ASFC Chemistry!Click the little i to the top right hand corner of this video to be taken to some of our other A-level chemist... Take R-22 Pressure-Enthalpy Diagram Figure 1-1, draw a condensing temperature line of 105ºF, an evaporative temperature line of -20ºF , constant throttling line from the 105ºF condensing liquid to -20ºF line to represent the expansion and draw the line of constant An enthalpy diagram allows us to easily see details of a chemical reaction. By knowing how to draw and label an enthalpy diagram we can see what the starting energy level is, how much energy is ...
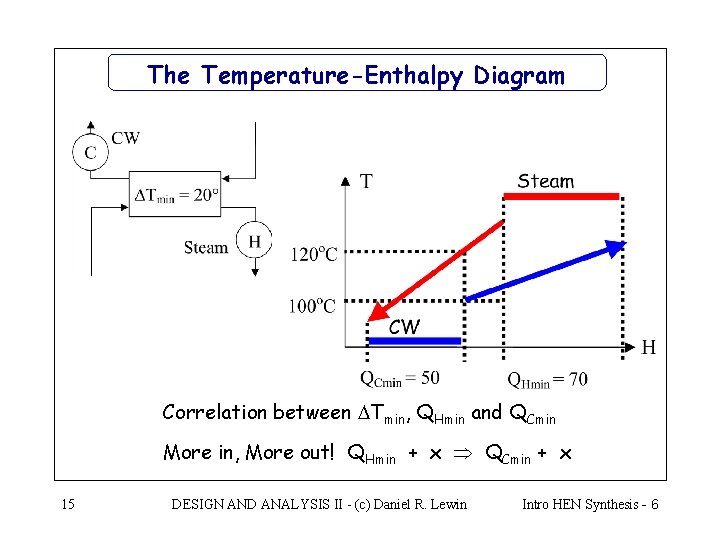
ENTHALPY-CONCENTRATION DIAGRAMS The variation of enthalpy for binary mixtures is conveniently represented on a diagram. An example is shown in Figure 3.3. The diagram shows the enthalpy of mixtures of ammonia and water versus concentration; with pressure and temperature as parameters.It covers the phase changes from solid to liquid to vapour, and the enthalpy values given include the latent ... How to Draw & Label Enthalpy Diagrams - Quiz & Worksheet. Choose an answer and hit 'next'. You will receive your score and answers at the end. Glucose + Oxygen goes to carbon dioxide and water ... A p-h diagram is a figure with a vertical axis of absolute pressure and a horizontal axis of specific enthalpy. It is an important diagram used frequently for a performance calculation of a refrigerating machine. A p-h diagram is made respectively for a specified refrigerant. It can, of course, not be used for another refrigerant. Enthalpy Diagram Background: Enthalpy diagrams are a great way to visualize changes in energy during a chemical reaction. Typically, the starting energy (reactants), ending energy (products ...
The change in enthalpy h is the enthalpy of the products the enthalpy of the reactants. Draw a curve 3 from point 1 parallel to an isentropic line is imp draw and label an enthalpy diagram that cooresponds to the given thermochemical equation 4fe 3o2 2fe2o3 1 65 x 10 3 kj. N2g h2g 2nh3g h 1003 kj.
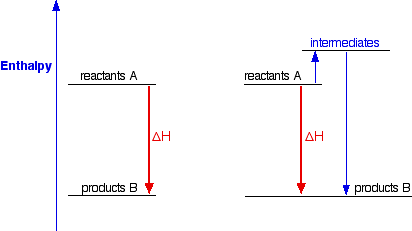
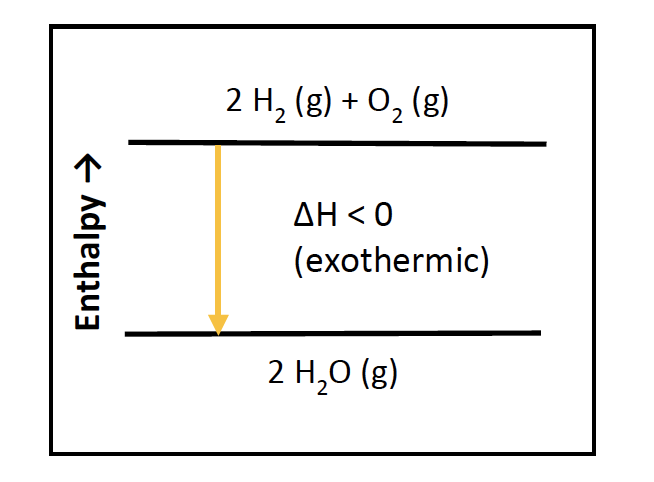
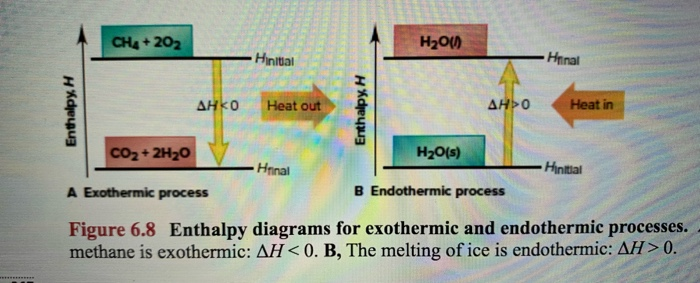
An enthalpy diagram shows the change in enthalpy of the reaction as the chemicals move from reactants to products. The change in enthalpy is a fancy term for the change in thermal energy of the system at constant pressure. Typically we classify reactions as either endothermic or endothermic. Endothermic means the thermal energy of the products is more than that of the reactants.
define. It is the ratio of heat content of a gas to its absolute temperature. It remains the same when a gas is compressed, if no heat is added or removed. When entropy is constant, the condition of the gas is called isentropic. SAMPLE DIAGRAMS . The most common type of pressure-enthalpy diagram is shown in Figures 1A through 1H. They
A simplified pressure-enthalpy diagram is shown below, describing this information. The curves break up the diagram into three regions (1) Liquid, (2) Vapor and (3) Mix. (1) Liquid Region: The liquid region is also known as the sub-cooled region. In this region there are vertical temperature lines, which increase as enthalpy is increased.
Mollier enthalpy-entropy chart for steam, US units. Image credit: Emok. The Mollier diagram is a tool used by engineers to predict, theoretically, the performance of systems and installations. The Mollier diagram, also called the enthalpy (h) - entropy (s) chart or h-s chart, is a graphical representation of thermodynamic properties of materials.
Enthalpy-concentration Diagram. M. Yuniardi. Enthalpy-concentration Diagram • McCabe Thiele method assumes constant molar flow rate because it considers equal latent heat of vaporization. • Here we consider varying molar flow rate by solving simultaneous material and energy balances. • In this case, the operating lines for the enriching ...
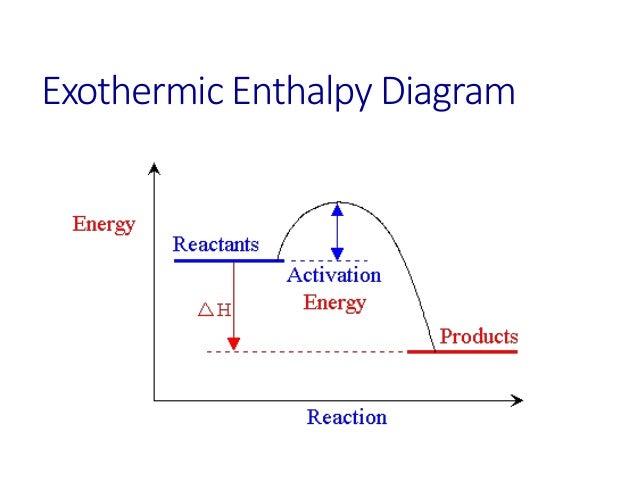
Enthalpy Diagrams. Enthalpy Profile Diagram This is the second set of enthalpy profile diagrams, these include the activation energy. Definition Activation energy (Ea) The minimum energy required for a reaction to occur. Factors that affect the rate of reaction 1. Catalyst 2. Temperature 3. Pressure for gases 4. Concentration for liquids 5.
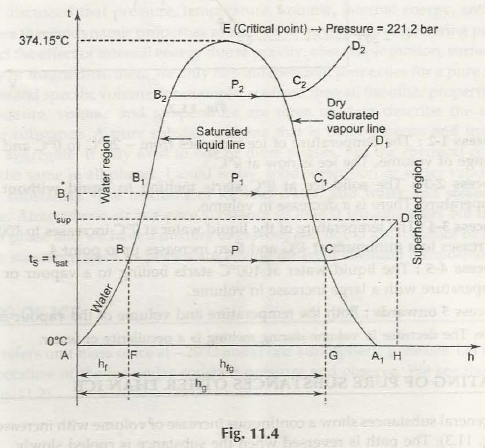
By the definition of entropy, the heat transferred to or from a system equals the area under the T-s curve of the process. Figure 13 is the T-s diagram for pure water. A T-s diagram can be constructed for any pure substance. It exhibits the same features as P-u diagrams. Temperature Entropy (T-s) Diagram
The enthalpy ( ∆H) is a measure of the actual energy that is liberated when the reaction occurs (the "heat of reaction"). If it is negative, then the reaction gives off energy, while if it is positive the ... Construction of an Ellingham Diagram An Ellingham diagram is a plot of ∆G versus temperature. Since ∆H and ∆S are essentially
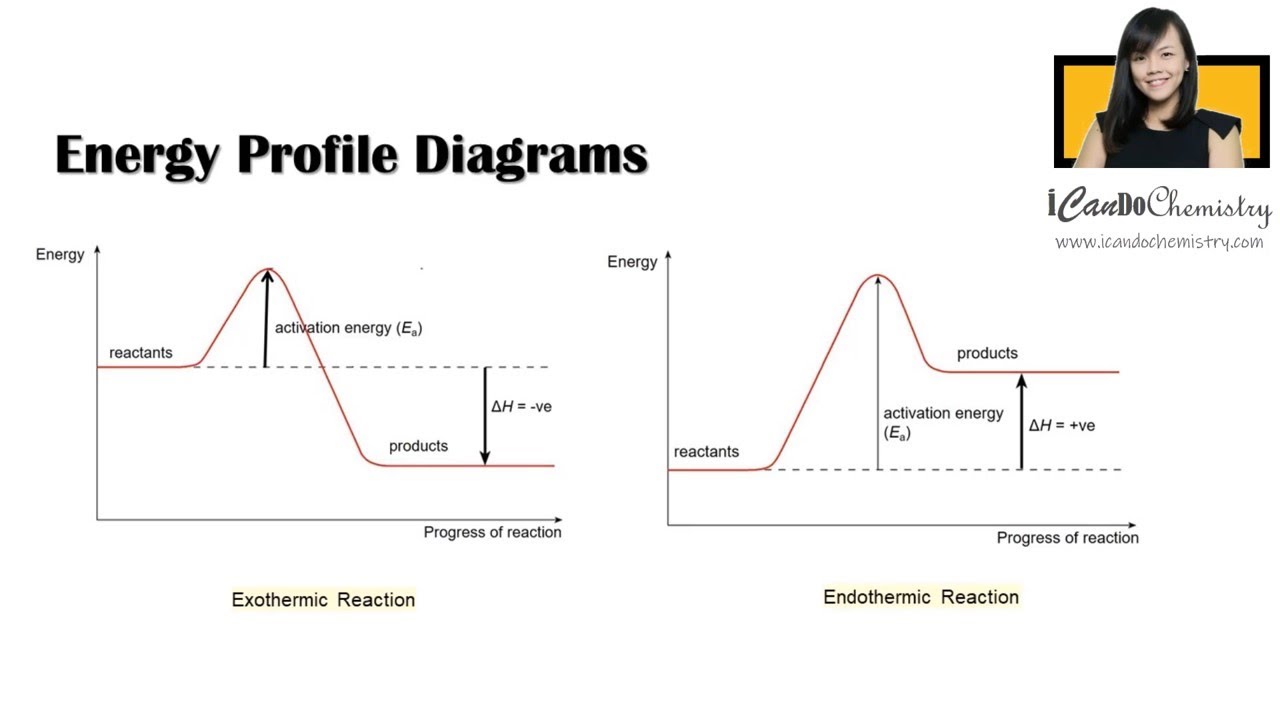
How To Draw Energy Profile Diagram And Energy Level Diagram Of Exothermic And Endothermic Reaction Online Video O Level Secondary Chemistry Tuition
Troubleshooting. 10. Systems. The pressure-enthalpy diagram (log P/h diagram) is a very useful tool for refrigerant technicians. First, an explanation of how the diagram is built up is given, and then its use is describ ed. Figure 2.1 shows the principle of a log P/h diagram, and indicates the refrigerant's various thermodynamic states.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
(In diagrams of this sort, we often miss off the standard symbol just to avoid clutter.) Then fit the other information you have onto the same diagram to make a Hess's Law cycle, writing the known enthalpy changes over the arrows for each of the other changes.
a) Draw an enthalpy level diagram for a neutralization reaction. i) Indicate on your diagram the enthalpy change of the reaction and deduce its sign. ii) Compare the relative stabilities and strengths of the bonds of the reactants and products. [4] iii) Define the term standard enthalpy change of a reaction. [1] 13.
Find step-by-step Chemistry solutions and your answer to the following textbook question: Draw an enthalpy diagram for a general exothermic reaction; label the axis, reactants, products, and $\Delta \mathrm { H }$ with its sign..

Draw An Energy Diagram For An Exothermic Reaction Label The Activation Enthalpy And The Change In Enthalpy Delta H On The Diagram Study Com
Sulphur Burns In Air To Form Sulphur Iv Oxide A Simple Energy Level Diagram For The Reaction Is Tutorke
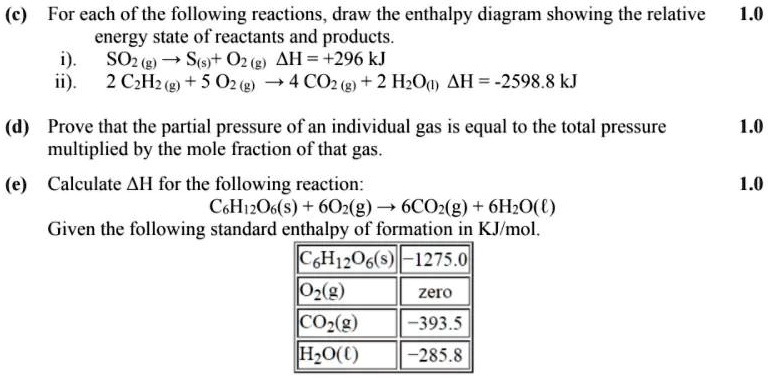
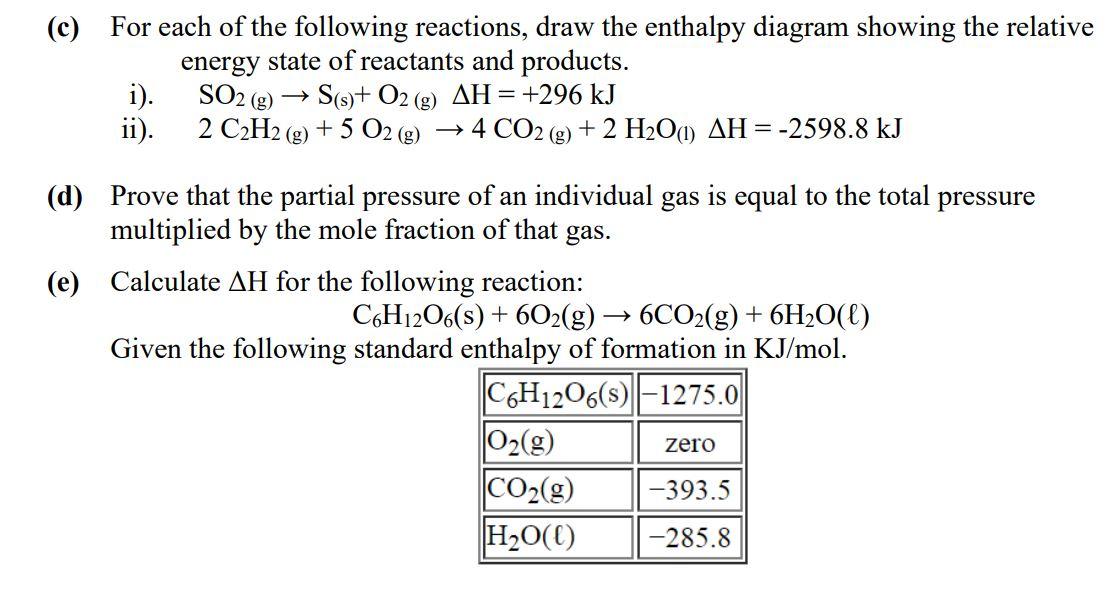
Solved C For Each Of The Following Reactions Draw The Enthalpy Diagram Showing The Relative Energy State Of Reactants And Products Soz G Sts 0z G Ah 296 Kj 2 Czhztg 5
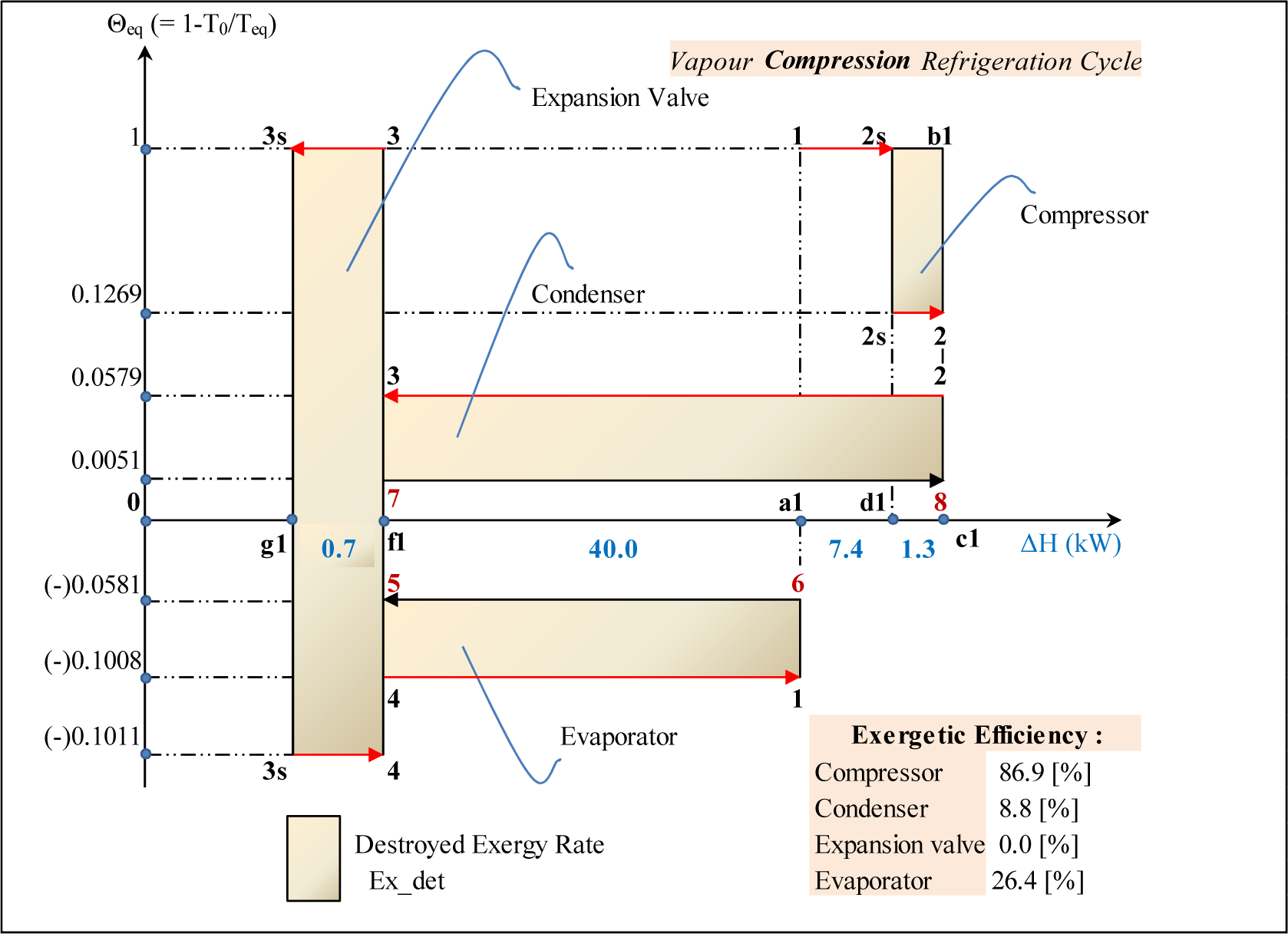
Entropy Free Full Text Equivalent Temperature Enthalpy Diagram For The Study Of Ejector Refrigeration Systems Html

Cambridge International As And A Level Chemistry Coursebook With Cd Rom By Cambridge University Press Education Issuu

A Level A Level 1 1 Advanced Introduction To Enthalpy Energy Changes Reaction Combustion Formation In Chemical Reactions Ks5 Gce Chemistry Revision Notes



















0 Response to "40 how to draw an enthalpy diagram"
Post a Comment