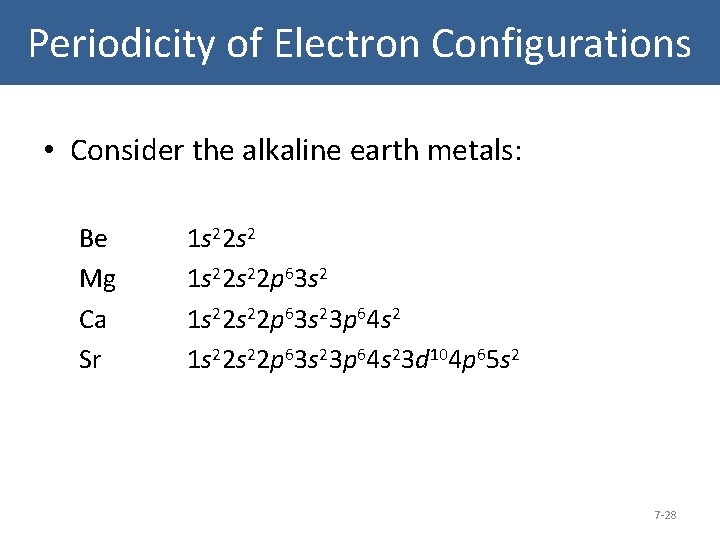
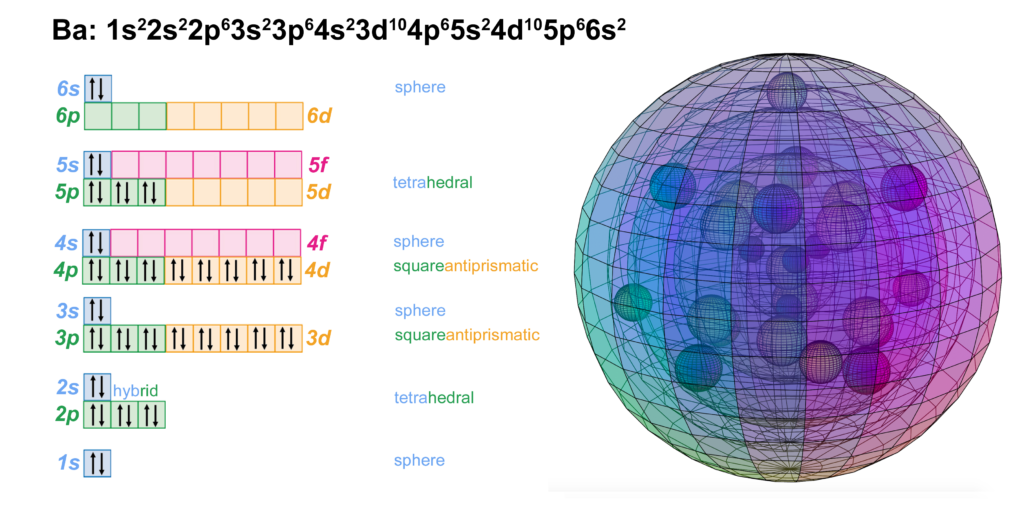
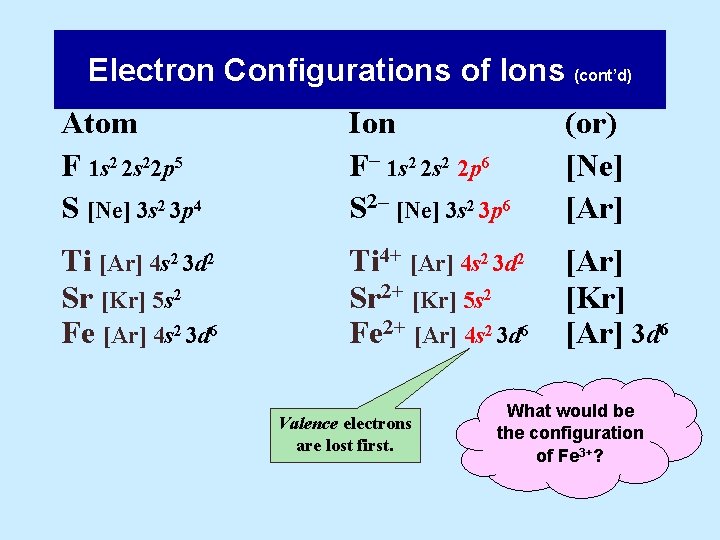
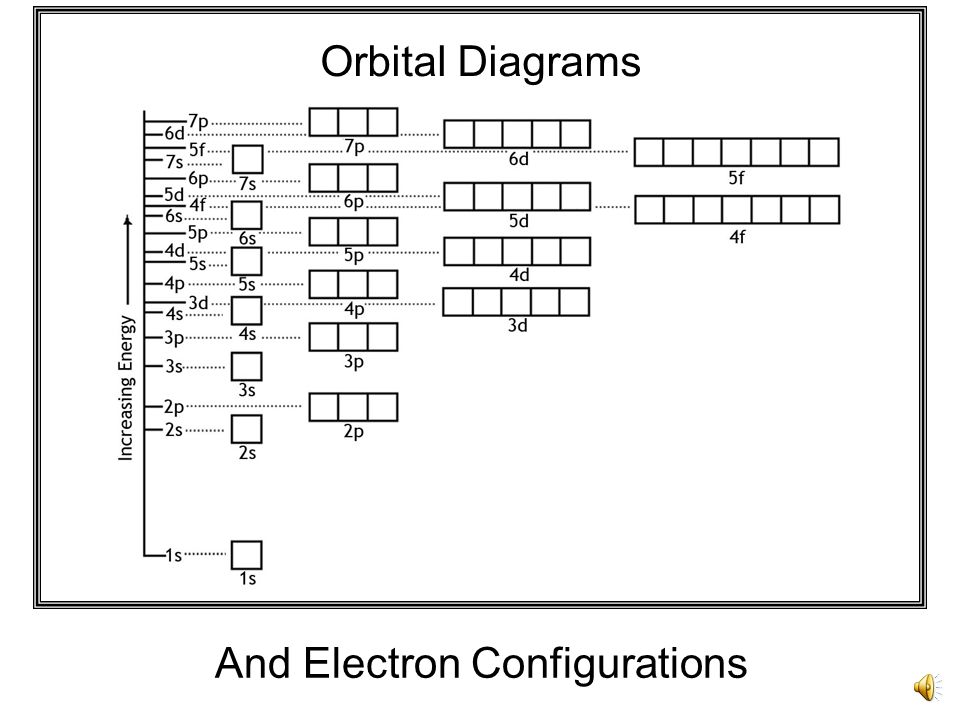
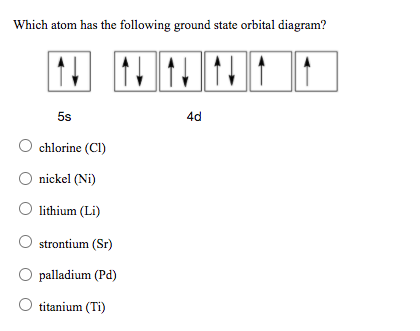
39 orbital diagram for strontium
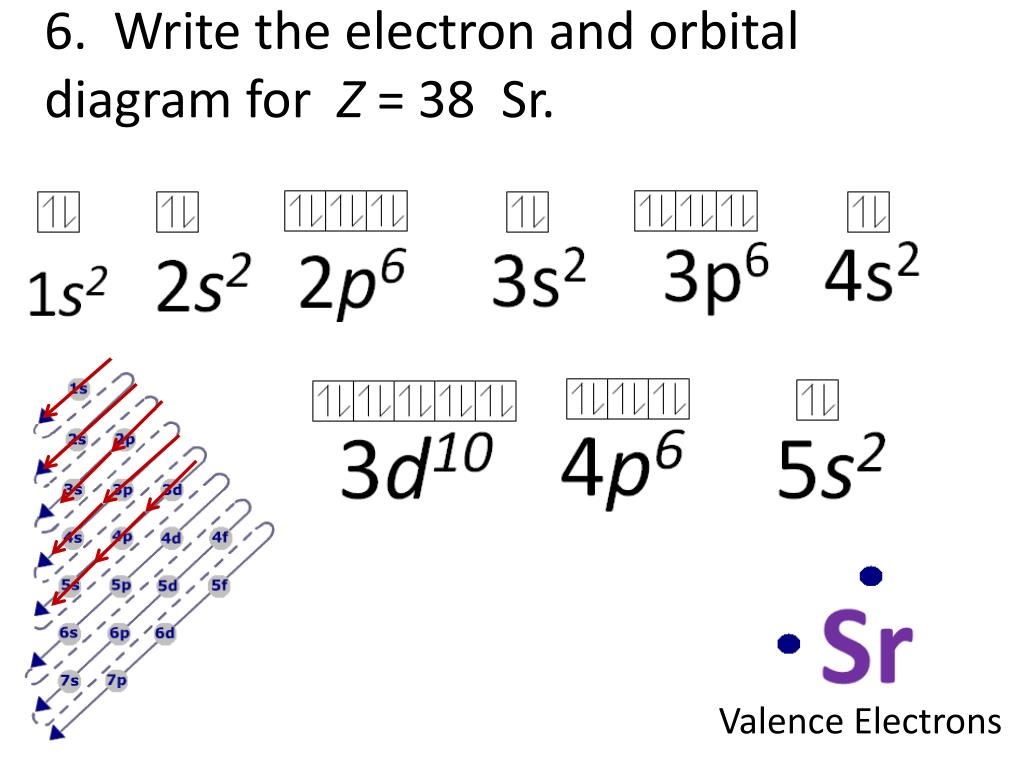
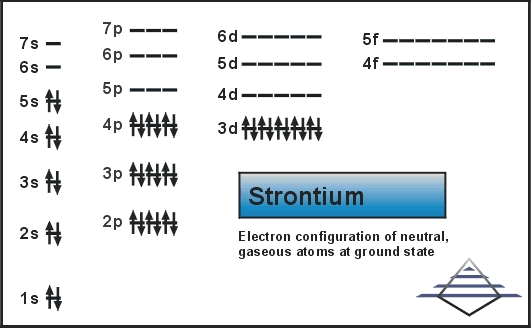
Strontium's full electron configuration is 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^6 5s2. Krypton has the electron configuration 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^6. These core electrons are stable and do not take part in bonding, while the remaining electrons are valence electrons. Orbital notation shows the number of electronics in an orbit. The orbital notation of Hydrogen is a circle with one slash through it. The electron configuration of Hydrogen is 1(s^1). Source: www ...
The electron configuration for strontium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 5s2, according to the Jefferson Lab website. The noble gas configuration of this element is [Kr] 5s2, with [Kr] representing the electron configuration of krypton. Strontium has an atomic number of 38, which means it has 38 protons. In a neutral atom, an equal number ...
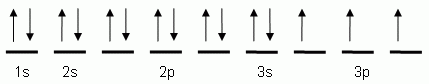
Orbital diagram for strontium
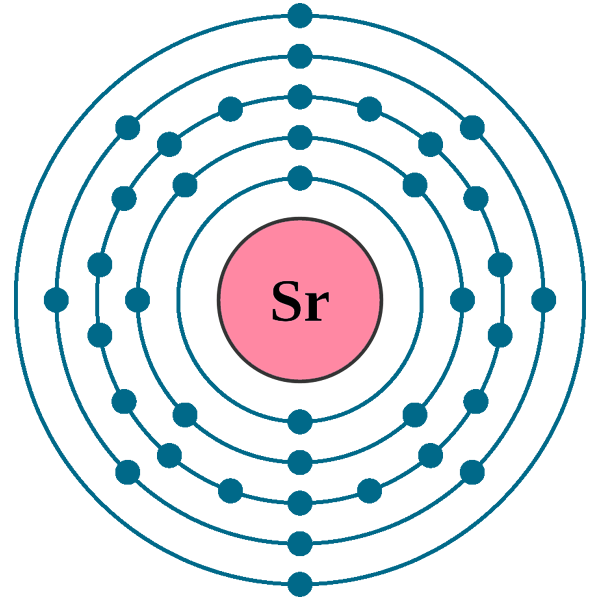
Strontium atoms have 38 electrons and the shell structure is 2.8. 18.8. 2. The ground state electron configuration of ground state gaseous neutral strontium is [Kr]. ... An orbital diagram helps to determine the electron configuration of an element. An element's electron configuration is the arrangement of the electrons in the shells. English: Electron shell diagram for Strontium, the 38th element in the periodic table of elements. Source: Own work: Author: Pumbaa (original work by Greg Robson) SVG development The SVG code is valid. This diagram was created with an unknown SVG tool (generated by script) PROBLEM: Use partial orbital diagrams and Lewis symbols to depict the formation of Na+ and O2! ions from the atoms, and determine the formula of the compound. Draw orbital diagrams for the atoms and then move electrons to make filled outer levels. Two sodiums are needed for each oxygen. 3s 3p Na 3s 3p Na 2s 2p O 2s p O2-2 Na+: Na Na + O ...
Orbital diagram for strontium. Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom Strontium-90, a radioactive isotope, is a by-product of nuclear reactors and present in nuclear fallout. It has a half-life of 28 years. It is absorbed by bone tissue instead of calcium and can destroy bone marrow and cause cancer. However, it is also useful as it is one of the best high-energy beta-emitters known. Selenium Orbital Diagram. here is the electronic configuration. Z=34 so 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p4. First, we consider a possibility of a double bond between the two selenium atoms . The corresponding molecular- orbital diagram for the Se 2 dimer, which is a. The orbital diagram is the most detailed picture of showing where all the To ... In order to write the Sr electron configuration we first need to know the number of electrons for the Sr atom (there are 38 electrons). When we write the c...
Start studying Honors Chemistry Unit 4 Test. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Use the orbital-filling diagram to show the electron configuration of helium, He. ... strontium phosphorus silver molybdenum. phosphorus germanium silver molybdenum strontium. Arrange the following elements in order of decreasing metallic character (high to low): Cl Cs Sr Rh Se Mo As Cd. Cs Sr Mo Rh Cd As Se Cl. The noble gas configuration of th the electron configuration for strontium is 1s2 2s2 2p6 3s strontium has an atomic n. Full Electron Configuration Of Calcium : Atomic Orbital Diagram of a Neutral Atom of Cesium - YouTube / The electron configuration for strontium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 5s2, according to the jefferson lab website ... Uses of Strontium: Used in flares and fireworks for crimson color. Also used in nuclear batteries in buoys and phosphorescent paint. Additional Notes: A. Crawford first recognized strontium as an element in 1790, but it wasn't isolated until 1808 by Sir Humphry Davy in London England. Strontium Menu. Strontium Page One. Overview of Strontium
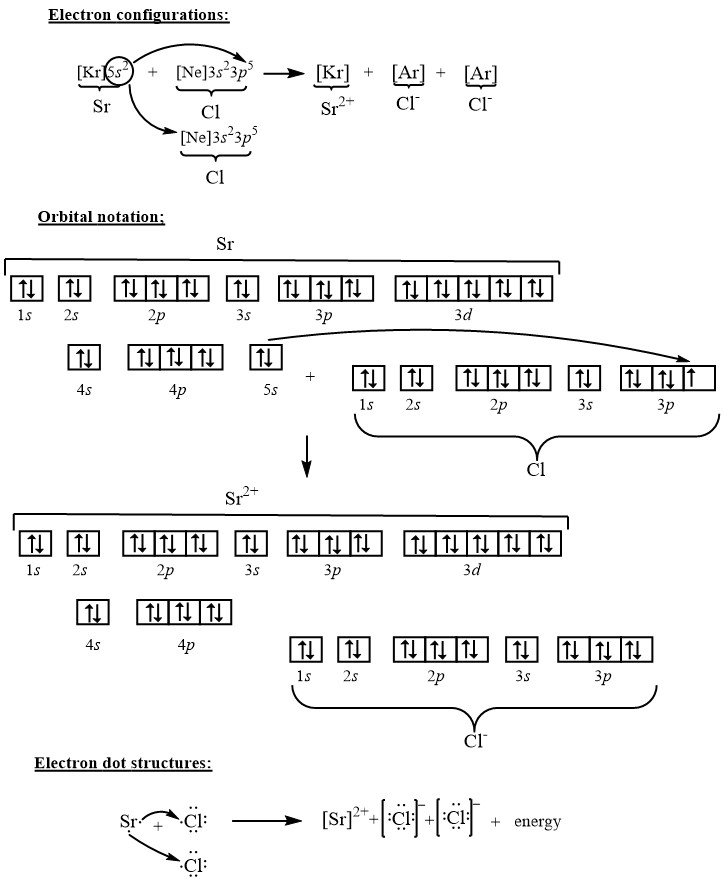
In an orbital (box) diagram a box represents each notation and an orbital diagram. strontium atom (a) in the spdf notation and (b) in the. In orbital notation this is: 1s22s22p63s23p63ds24p65s2. The noble gas shorthand notation is: [Kr]5s2. When strontium forms a 2+ ion the 2. Oxidation States, 2. Electrons Per Shell, 2 8 18 8 2. Electron binding energies for strontium. All values of electron binding energies are given in eV. The binding energies are quoted relative to the vacuum level for rare gases and H 2, N 2, O 2, F 2, and Cl 2 molecules; relative to the Fermi level for metals; and relative to the top of the valence band for semiconductors. Label Orbital eV ... We thoroughly check each answer to a question to provide you with the most correct answers. Found a mistake? Let us know about it through the REPORT button at the bottom of the page. n atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other … Electron Configuration Practice Quiz Read More » When strontium and chlorine form an ionic compound, strontium, the metal is going to donate its electrons to form a cat eye on and chlorine, a nonmetal is going to accept the electrons to form an an eye on. We can describe this process three different ways. Using electron configurations, orbital notation and electron dot structures, strontium has an electron configuration.
Nov 01, 2021 · Orbital diagram of Strontium (Sr) 39: Orbital diagram of Yttrium (Y) 40: Orbital diagram of Zirconium (Zr) 41: Orbital diagram of Niobium (Nb) 42: Orbital diagram of Molybdenum (Mo) 43: Orbital diagram of Technetium (Tc) 44: Orbital diagram of Ruthenium (Ru) 45: Orbital diagram of Rhodium (Rh) 46: Orbital diagram of Palladium (Pd) 47: Orbital ...
In an orbital (box) diagram a box represents each notation and an orbital diagram. strontium atom (a) in the spdf notation and (b) in the. Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of strontium (atomic number: 38), the most. Oxidation States, 2. Electrons Per Shell, 2 8 18 8 2.
15.11.2021 · The electron configuration and orbital diagram for carbon are: Nitrogen (atomic number 7) fills the 1 s and 2 s subshells and has one electron in each of the three 2 p orbitals, in accordance with Hund’s rule. 2-6-1 **Note the # of electrons are the same. * 2s 2 2p 3 configuration is more stable than 2s 2 2p 4 due to half filled p-sublevel.
7. [-14 Points] DETAILS PREPCHEMAF1 4.AP. 114. Which sublevel contains: (a) the highest-energy electron for strontium, Sr? (b) the 63rd electron added to an orbital diagram for elements larger than samarium, Sm? (c) the 33rd electron added to an orbital diagram for elements larger than germanium, Ge? (d) the 75th electron added to an orbital ...
Interactive 3D chemistry animations of reaction mechanisms and 3D models of chemical structures for students studying University courses and advanced school chemistry ...
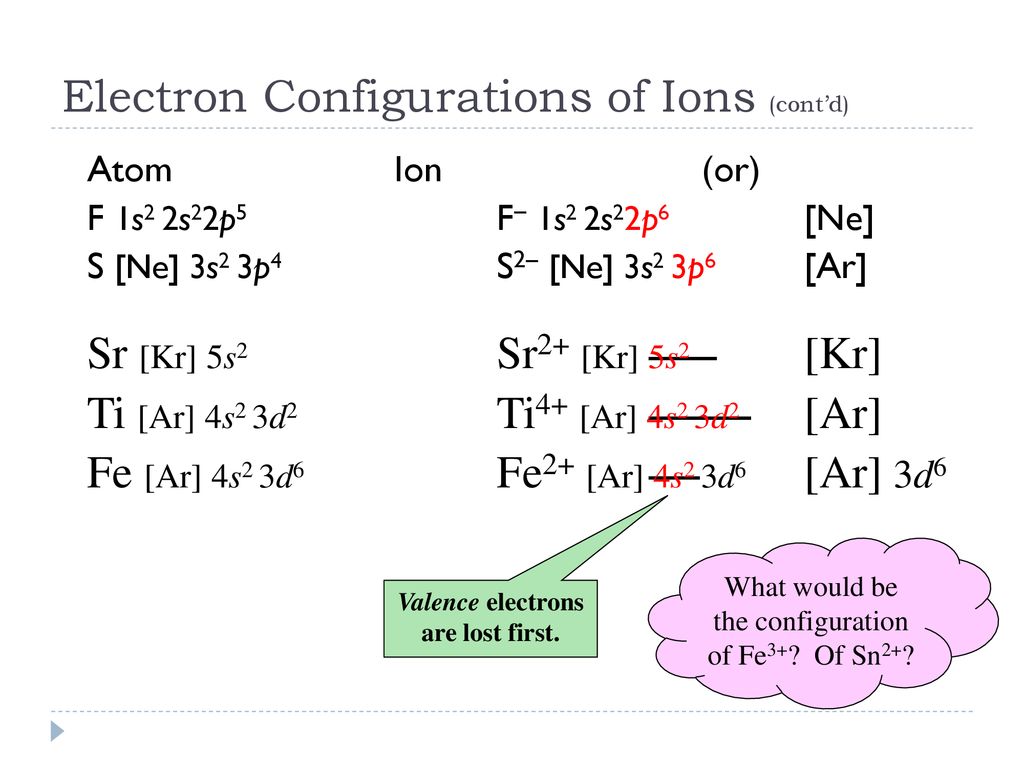
Strontium is atomic number 38 so its electron configuration can be written: 2.8.18.8.2. In orbital notation this is: 1s22s22p63s23p63d104s24p65s2. The noble gas shorthand notation is: [Kr]5s2. When strontium forms a 2+ ion the 2 outer 5s electrons are lost so Sr2+ can be written as: 1s22s22p63s23p63d104s24p6.
Electronic configuration of the Strontium atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 Electronic configuration of the Strontium atom in ascending order of the levels: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 5s 2 Reduced electronic configuration Sr: [Kr] 5s 2
Orbital Diagram. 1s ... Soft, malleable, silvery-yellow metal. Uses Used in flares and fireworks for crimson color. Strontium-90 is a long lived highly radioactive fallout product of atomic-bomb explosions. Sources Found in minerals celestite and strontianite. Pronounciation (English) STRON-she-em Translations. Afrikaans
The electron configuration for strontium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 5s2, according to the jefferson lab website. Examples include neon, hydrogen, and argon. Paper boat creative/getty images an atom is the basic unit of an element. ... Atomic Orbital Diagram of a Neutral Atom of Zinc ...
Strontium Orbital Diagram. In orbital notation this is: 1s22s22p63s23p63ds24p65s2. The noble gas shorthand notation is: [Kr]5s2. When strontium forms a 2+ ion the 2. Comprehensive information for the element Strontium - Sr is provided by this page Comprehensive data on the chemical element Strontium is provided on this. Oxidation States, 2.
In an orbital diagram, such as the one below, each small box represents which of the following? Each box represents an individual orbital, ... Strontium's full electron configuration is 1s22s22p63s23p64s23d104p65s2. Krypton has the electron configuration 1s22s22p63s23p64s23d104p6.
Fluorine electron configuration is 1s 2 2s 2 2p 5.The symbol for fluorine is F. The period of fluorine is 2 and it is a p-block element. The electron configuration of fluorine(F) and the orbital diagram is the main topic of this article.
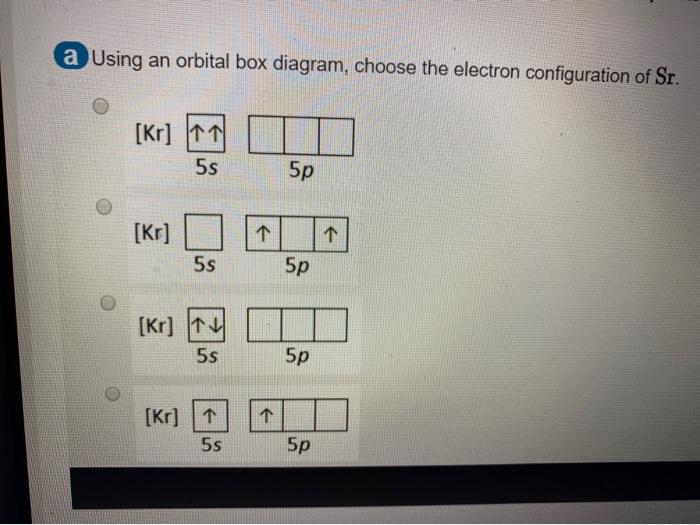
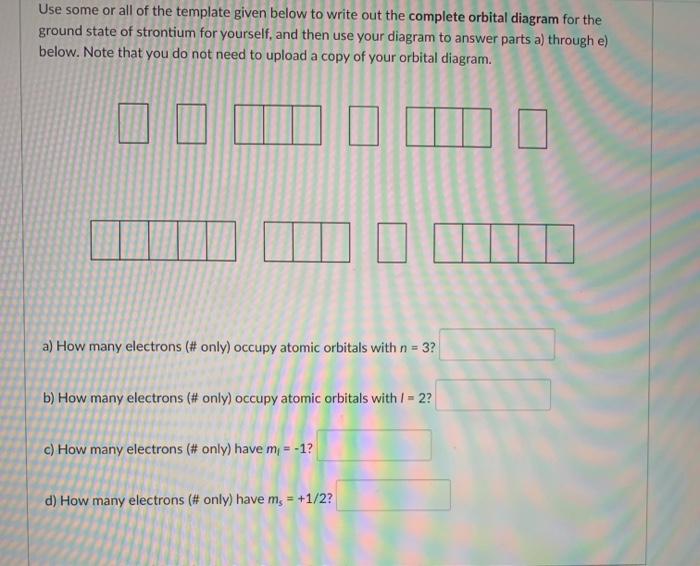
For Strontium:a) Write the full electron configuration.b) Write the condensed electron configuration.c) Predict the common ion for Strontium.d) Write the con...
Electron configuration was first conceived under the Bohr model of the atom, and it is still common to speak of shells and subshells despite the advances in understanding of the quantum-mechanical nature of electrons.. An electron shell is the set of allowed states that share the same principal quantum number, n (the number before the letter in the orbital label), that electrons may occupy.
The orbital diagram for hydrogen can be represented in the following way. This notation uses a box to represent the orbital, the label for the orbital and an arrow to represent the electron. The electronic configuration for hydrogen can be written as 1s 1. This is a short-hand notation which identifies the level, the sublevel and the number of ...
orbital diagrams for copper shows that the 3 d orbitals in the experimental diagram are completely filled, whereas, in the theoretical diagram they are not. Therefore, completely filling the 3 d orbitals must confer stability on the electron configuration more than does filling the s orbital. In summary, w hen the 3 d orbitals are either all half-
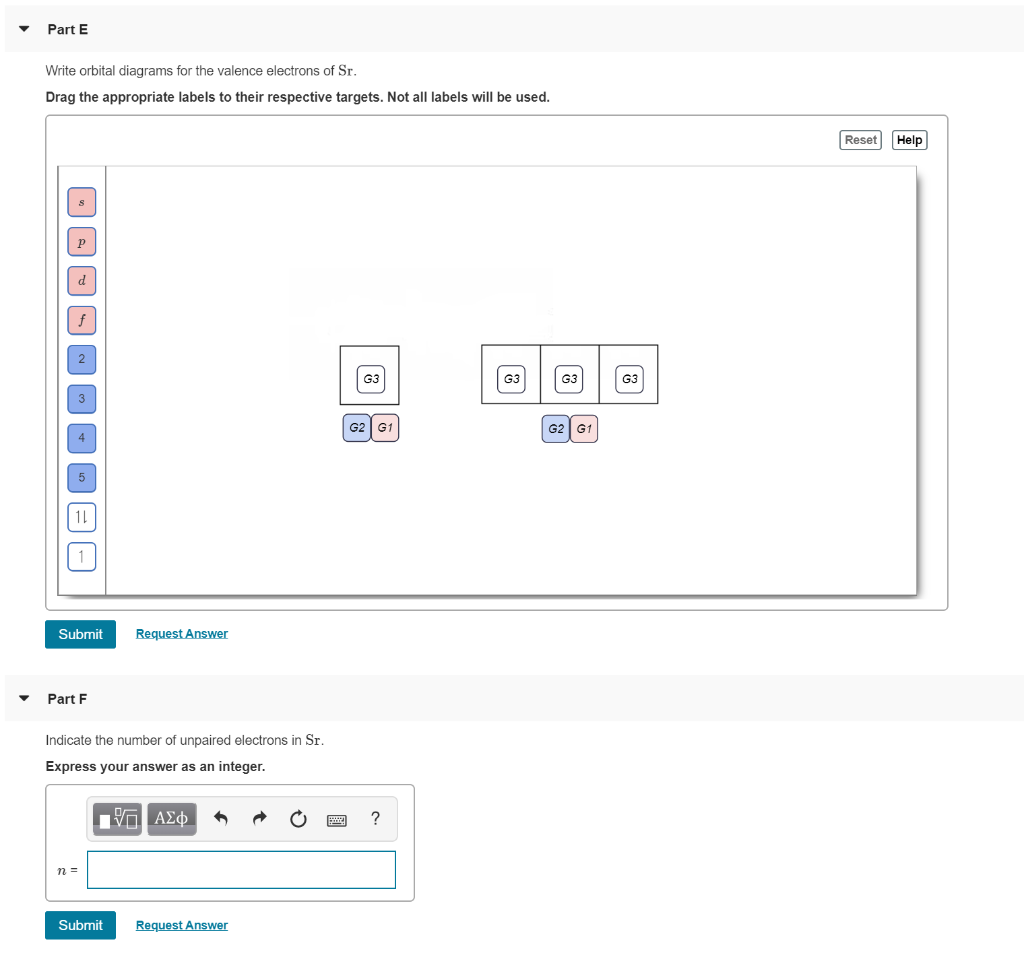
Chemistry Q&A Library What is the orbital diagram for the atom Strontium Sr 38. What is the orbital diagram for the atom Strontium Sr 38. close. Start your trial now! First week only $4.99! arrow_forward. Question. What is the orbital diagram for the atom Strontium Sr 38. check_circle Expert Answer.
A geostationary orbit, also referred to as a geosynchronous equatorial orbit (GEO), is a circular geosynchronous orbit 35,786 kilometres (22,236 miles) in altitude above Earth's equator (42,164 kilometers in radius from Earth's center) and following the direction of Earth's rotation.. An object in such an orbit has an orbital period equal to the Earth's rotational period, one sidereal day, and ...
20.2.2021 · Carbon Electron Configuration: If you guys have come across our recent article then it would be easy for you all to understand the concept.But if you are new here and looking for the information related to the carbon element and its electronic configuration, then today we will help you with some of the things and if you will be here till the last line surely you will go with some knowledge ...
PROBLEM: Use partial orbital diagrams and Lewis symbols to depict the formation of Na+ and O2! ions from the atoms, and determine the formula of the compound. Draw orbital diagrams for the atoms and then move electrons to make filled outer levels. Two sodiums are needed for each oxygen. 3s 3p Na 3s 3p Na 2s 2p O 2s p O2-2 Na+: Na Na + O ...
English: Electron shell diagram for Strontium, the 38th element in the periodic table of elements. Source: Own work: Author: Pumbaa (original work by Greg Robson) SVG development The SVG code is valid. This diagram was created with an unknown SVG tool (generated by script)
Strontium atoms have 38 electrons and the shell structure is 2.8. 18.8. 2. The ground state electron configuration of ground state gaseous neutral strontium is [Kr]. ... An orbital diagram helps to determine the electron configuration of an element. An element's electron configuration is the arrangement of the electrons in the shells.
Solved Construct The Molecular Orbital Diagram For Srcl Would You Expect The Bond Length Of Mathrm Srcl To Be Longer Or Shorter Than That Of Srcl





















0 Response to "39 orbital diagram for strontium"
Post a Comment