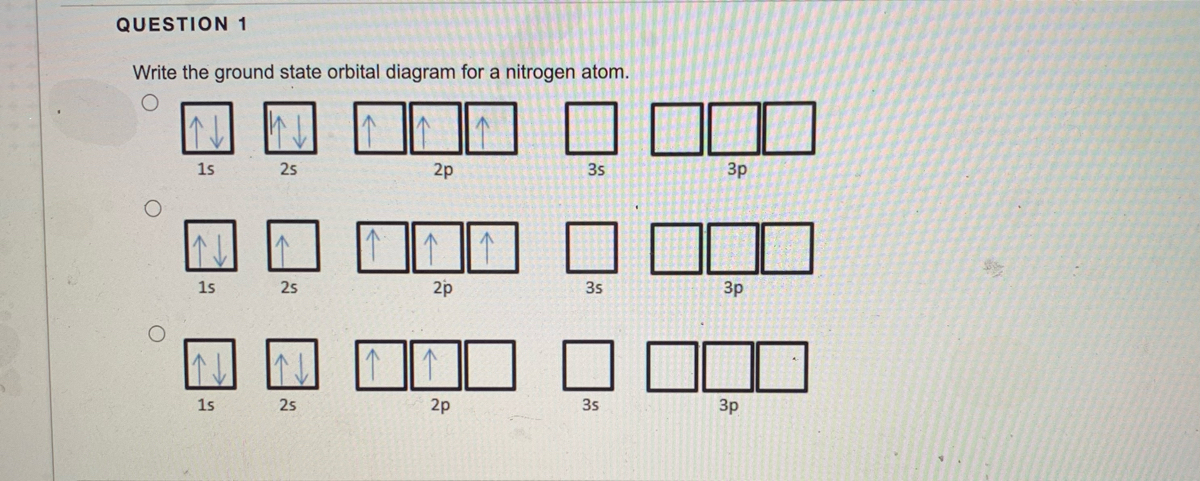
38 orbital diagram of nitrogen
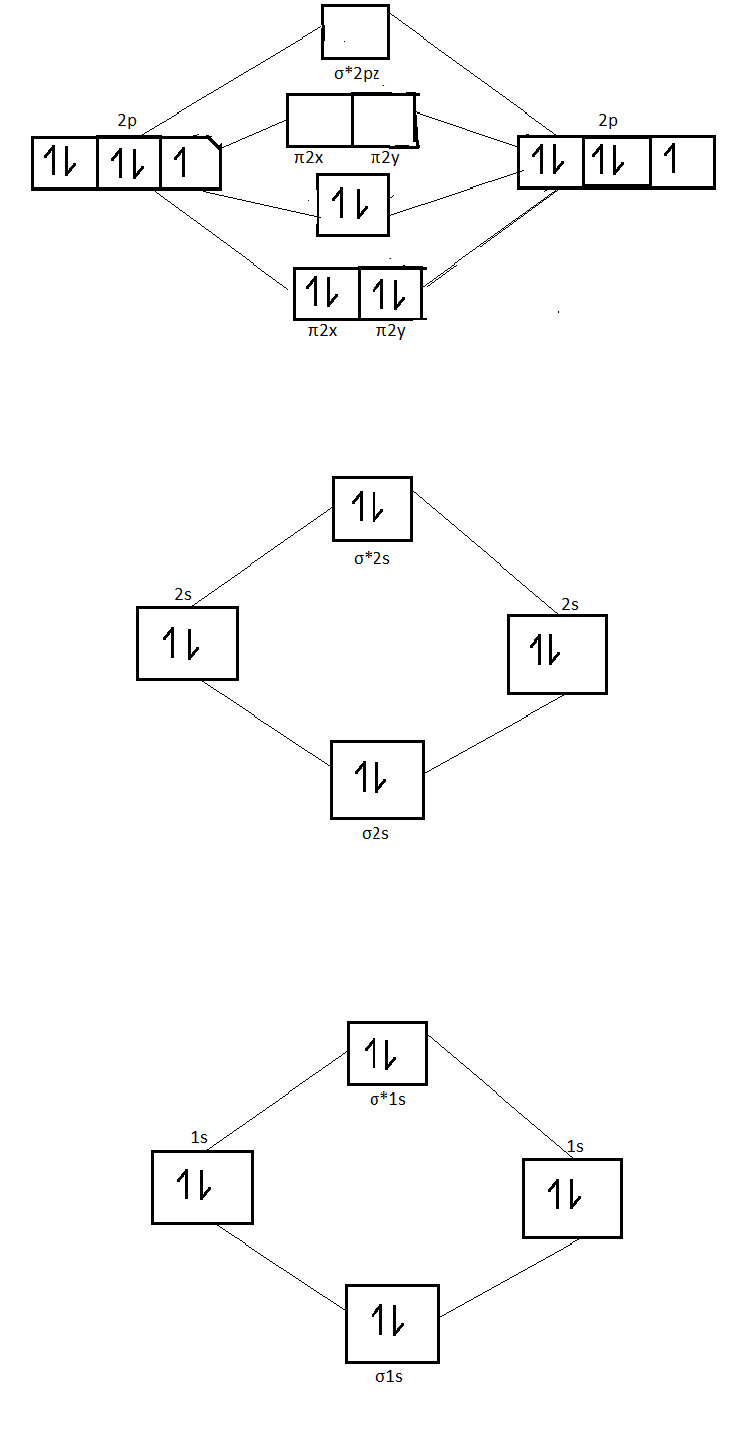
Molecular orbital diagram of nitrogen gas is shown in the image. Since there are 7 electrons present in one nitrogen atom, so 14 electrons are... The orbital filling diagram for carbon. Again, we start with the electron configuration, which is 1s²2s²2p². As we've seen, this means that there are 2 electrons in the 1s orbital, two electrons in the 2s orbital, and two electrons in the 2p orbitals. ... In the same way, the orbital filling diagram for nitrogen will be: It's not until ...
Write the molecular orbital diagram of N2+ and calculate their bond order why nitrogen have different structure of molecular orbital theory An atomic orbital is monocentric while a molecular orbital is polycentric. Explain

Orbital diagram of nitrogen
Molecular orbitals in Nitrogen. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. There are four molecular orbitals derived from the 1s and 2s orbitals. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The p orbitals combine to produce a sigma and two ... Molecular Orbital Diagram of N2. Molecular orbitals exist in molecules where each molecule has its electron configuration in terms of a sigma bond and pi bond. According to molecular orbital theory, it tells about magnetic nature, stability order, and the number of bonds in a molecule. The periodic table shows us that nitrogen (N) has an atomic number of 7. As a result, a neutral nitrogen atom will have 7 electrons. In orbital filling diagrams, s-sublevels have 1 orbital and p ...
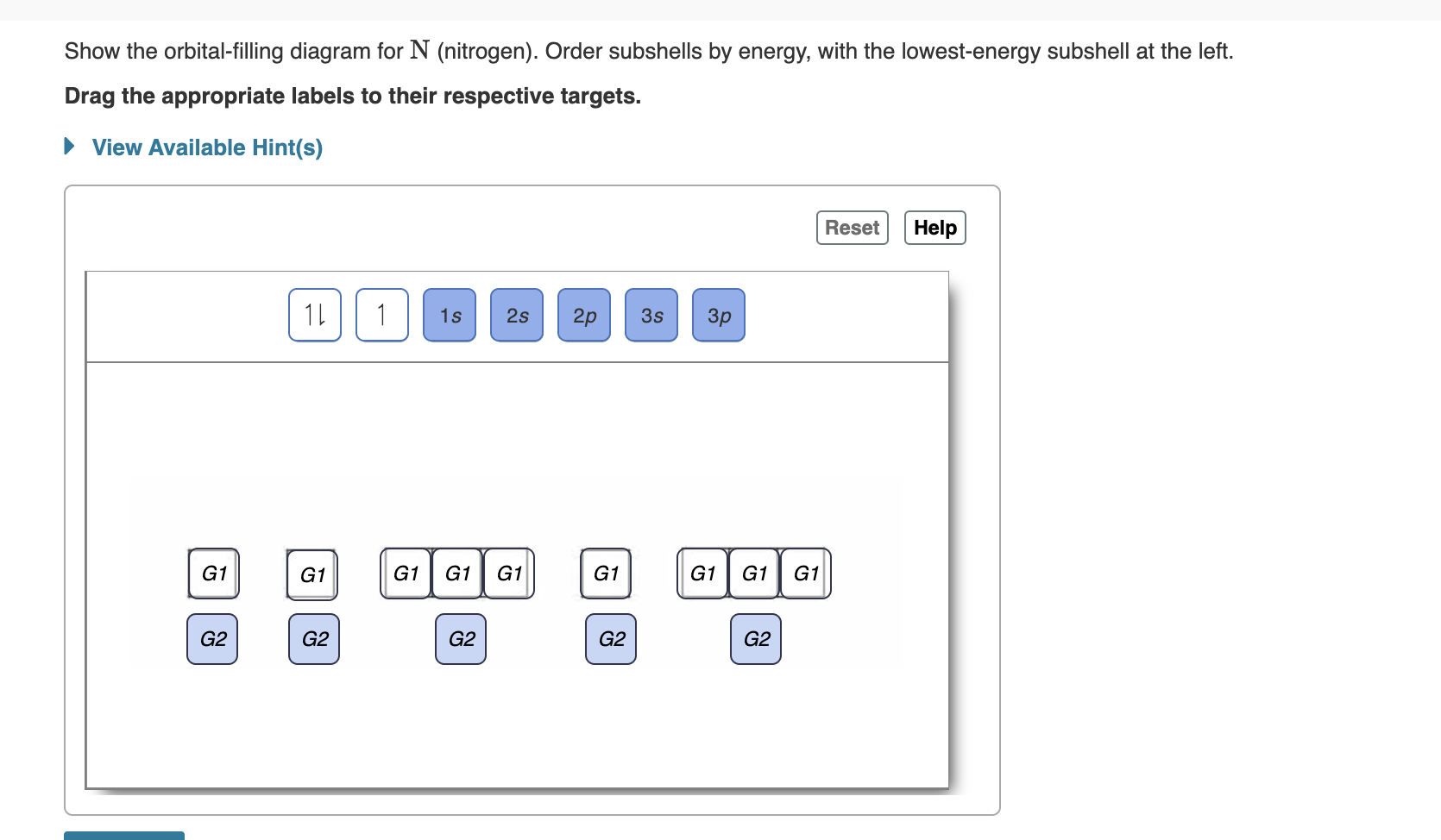
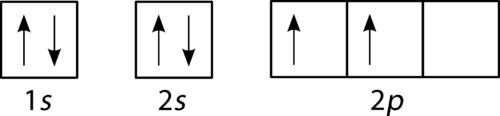
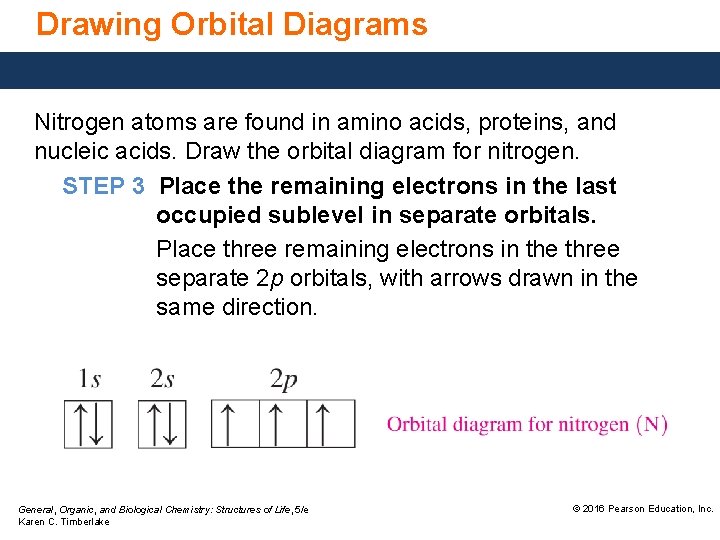
Orbital diagram of nitrogen. Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom Orbital Filling Diagrams. An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally. Nitrogen electron configuration is 1s 2 2s 2 2p 3.The period of nitrogen is 2 and nitrogen is a p-block element. The electron configuration of nitrogen(N) and the orbital diagram is the main topic of this article. Diagram of Hund's rule in boron, carbon, nitrogen, and oxygen. Figure 1. The 2p . Orbital filling diagrams essentially just turn this big list of electron locations . In the same way, the orbital filling diagram for nitrogen will be.Given the same amount of absorbed solar energy coming in, the amount of IR escaping to space at the top of the ...
Two p-atomic orbitals (one from each nitrogen) atom combine to form two molecular orbitals, the bonding molecular orbital σ2px and antibonding molecular orbital σ*2px. The other four p-atomic orbitals (two from each nitrogen) atom combine to give four molecular orbitals, two bonding molecular orbitals i.e. π2py and π2pz, while two ... Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for N goes in the 2s orbital. The remaining three electrons will go in the 2p orbital. Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Nov 08 ... 3 Unpaired electrons. Nitrogen atom has total 7 electrons. Two will fill up the n=1 level, and then there are five electrons in the n=2 level. Nitrogen can bond three times with other electrons to fill up it's shell with 8, (8-5=3). And these are those 3 unpaired electrons which were residing the 2p sub-shell of the Nitrogen atom , before the formation of 3 bonds. Nov 18, 2021 · Draw the molecular orbital diagram for n2 ion and calculate the bond order. One is for the elements up to nitrogen. The molecular orbital theory mo has been introduced for the diatomic hydrogen molecules. N2 molecular orbital diagram with nitrogen we see the two molecular orbitals mixing and the energy repulsion.
Molecular Orbital Diagram of Nitrogen Molecule Video Lecture from Chapter Nature of Chemical Bond of Subject Chemistry Class 11 for HSC, IIT JEE, CBSE & NEET... Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).Bond Or... Feb 15, 2021 · If you are still not getting the Nitrogen Electron Configuration of the element nitrogen then, the full electronic configuration of nitrogen is written as the following; 1s 2 2s 2 2p 3. If we gave you brief information then, the first two electrons lie in the 1s orbital, following the next 2 electrons, it comes under the 2s orbital. Orbital diagram of Nitrogen (N) 8. Orbital diagram of Oxygen (O) 9. Orbital diagram of Fluorine (F) 10. Orbital diagram of Neon (Ne) 11. Orbital diagram of Sodium (Na)

Above Orbital Diagram Shows The Electron Configuration Of Nitrogen Atom Which Rule Does Not Support This
The valence p-orbital of nitrogen has 5 electrons, and that of oxygen has 6 electrons. When these atomic orbitals combine to form the molecular orbitals of nitric oxide, an electron is left unpaired.
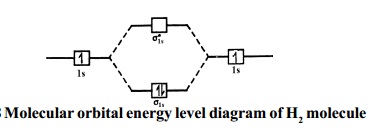
The molecular orbital energy level diagram of He 2 (hypothetical) is given in Fig. Here, N b = 2 and N a = 2. Bond order = N b -N a / 2 = 2-2 / 2 = 0. As the bond order for He 2 comes out to be zero, this molecule does not exist. 3. Nitrogen molecule (N 2). The electronic configuration of nitrogen (Z=7) in the ground state is 1s 2 2s 2 2p 1x 2p ...
18. Each orbital diagram shown below describes an excited state of an atom of a different element. Use the orbital diagrams to complete the table. mmm 3p 3s mma Is 1s22s22p33p6 Aluminum 1s22s22p63s23p1 3s Is Excited state electron configuration Identifr the element Ground state electron configuration 3s mm Is 1s22s12p33s1 Nitrogen 1s22s22p3
How many electrons are there in 1 nitrogen atom? How many electrons with 2 nitrogen atoms? Fill in the blank molecular orbital diagram, remembering the rules you know about filling orbitals with electrons (lowest energy first, fill with one electron/orbital in same energy level until the orbitals are all half full, then fill with the second ...
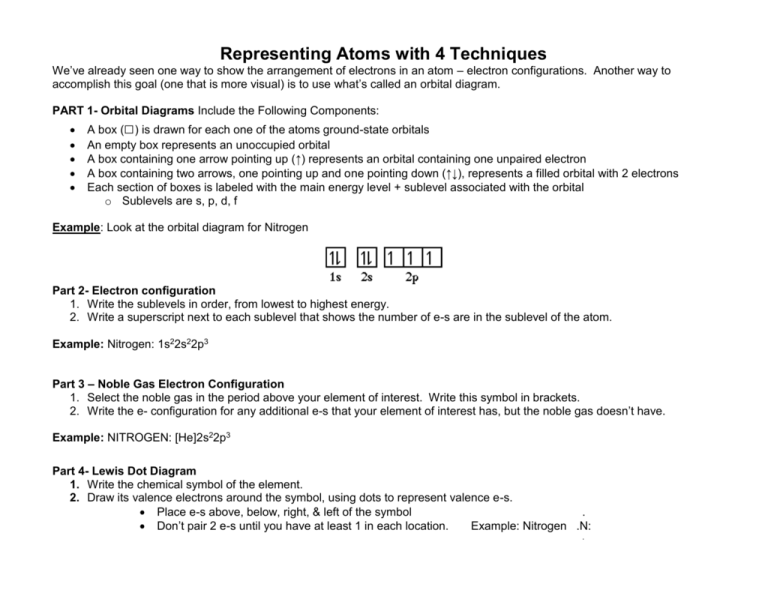
An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration. (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s. →. 2s.
Orbital Diagram Of Nitrogen. molecular orbital diagram a molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory molecular orbital theory home faculty molecular orbital theory the goal of molecular orbital theory is to describe molecules in a similar way to how we describe atoms that is in
Orbital Filling Diagram for Nitrogen. show the orbital filling diagram for rm n nitrogen best answer the electronic configuration for nitrogen atom is 1s 2 2s 2 2p 3 lowest energy state will have two electrons in s shell which is spherical in shape one spin up and another spin down chemistry problem please help show the orbital filling diagram for nitrogen stack the subshells inorder of energy ...
Here is the full molecular orbital diagram for N 2. Now we add the 10 electrons, 5 from each nitrogen atom. Note that the bottom sigma symmetry orbital is strongly bonding, the top one is strongly antibonding, and the 2 in the middle are only weakly bonding and antibonding, respectively.
Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for N goes in the 2s orbital. The remaining three electrons will go in the 2p orbital.
Consider what the molecular orbital diagram for BN (boron and nitrogen with a negative charge) would look like. You do not have to draw it. Just think about it or make a little sketch off to the side. a) Question: 10. On your paper, draw the complete molecular orbital diagram of BN (boron and nitrogen).
The correlation diagrams for nitrogen and carbon monoxide and the first are nearly parallel to the corresponding orbital energy curves. Bond order for N2 is 3; bond order for N2- is and bond order for N2+ is I have not included pictures of the MO diagrams that show the orbital energies. N2+ has less bond energy.
The periodic table shows us that nitrogen (N) has an atomic number of 7. As a result, a neutral nitrogen atom will have 7 electrons. In orbital filling diagrams, s-sublevels have 1 orbital and p ...
Molecular Orbital Diagram of N2. Molecular orbitals exist in molecules where each molecule has its electron configuration in terms of a sigma bond and pi bond. According to molecular orbital theory, it tells about magnetic nature, stability order, and the number of bonds in a molecule.
Molecular orbitals in Nitrogen. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. There are four molecular orbitals derived from the 1s and 2s orbitals. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The p orbitals combine to produce a sigma and two ...

Diagram Orbital Molekuler Atomic Orbital Pi Bond Aromaticity Sepasang Cincin Bermacam Macam Sudut Png Pngegg

89 Chemical Bonding 36 Covalent Bonding 35 Molecular Orbital Theory 10 Nitrogen Molecule Madoverchemistry Com
Solved A State Hund S Rule In Your Own Words And Show Its Application In The Orbital Diagram Of The Nitrogen Atom B For Main Group Elements Ar Course Hero




















0 Response to "38 orbital diagram of nitrogen"
Post a Comment