38 e1 reaction coordinate diagram
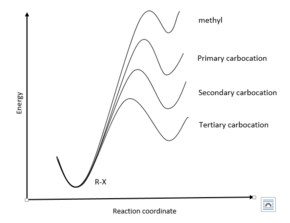
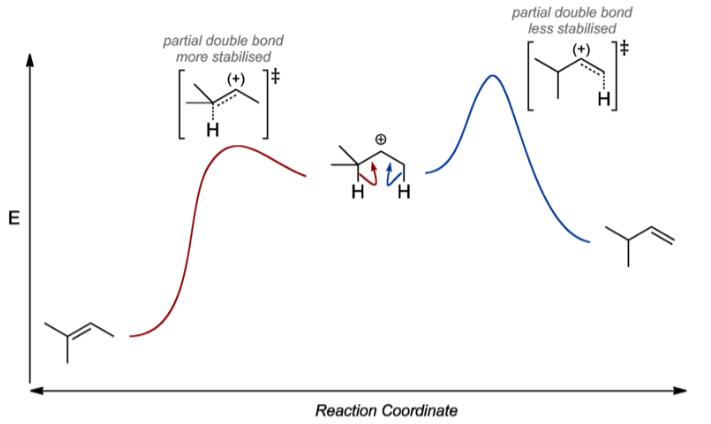
Increasing rate of E1 reaction RCH 2 XR 2CH XR 3C X 1° 2° 3° + + + I i bili f b i RCH 2 R 2CH R 3C 1° 2° 3° ncreas ng sta ty o car ocat ons The strength of the base usually determines whether a reaction follows the E1 or E2 mechanism. Strong bases like OH−and OR−favor E2 reactions, whereas weaker bases like H 2 O and ROH favor E1 ... Potential Energy Diagram for E1 TS energy depends on carbocation rate-determining titi tt stability and leaving group quality. (Same as SN1.) H CH3 CH3 E transition state TS energy does not depend on the strength of the base. X H H a rate determining E - step reaction coordinate E1 and SN1 Frequently Occur Together (because they pass through a common intermediate)
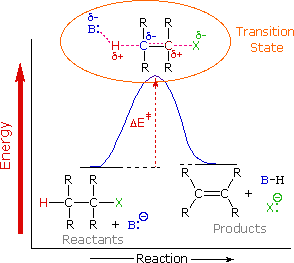
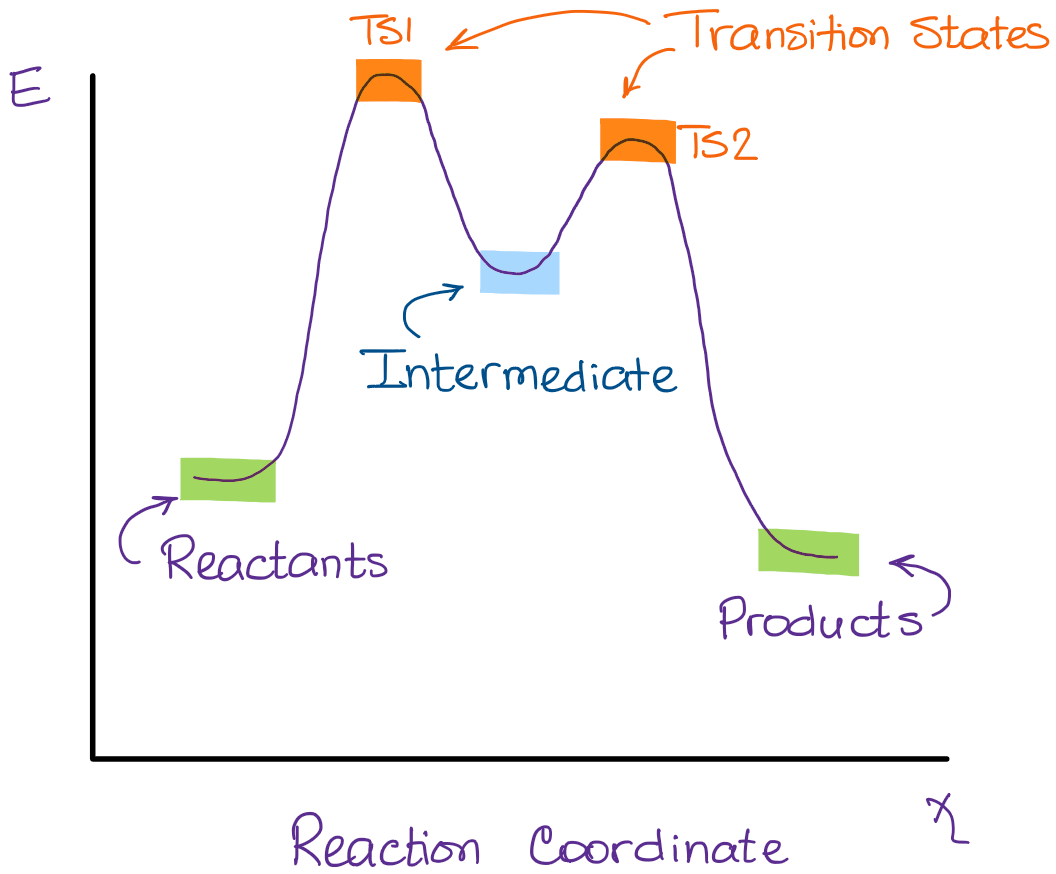
The following simple reaction coordinate diagram provides a basis for the key issues about kinetic and thermodynamic control: Consider the case where a starting material, SM , can react in a similar manner to give two different products, P1 and P2 via different (competing) pathways represented by green and blue curves.
E1 reaction coordinate diagram
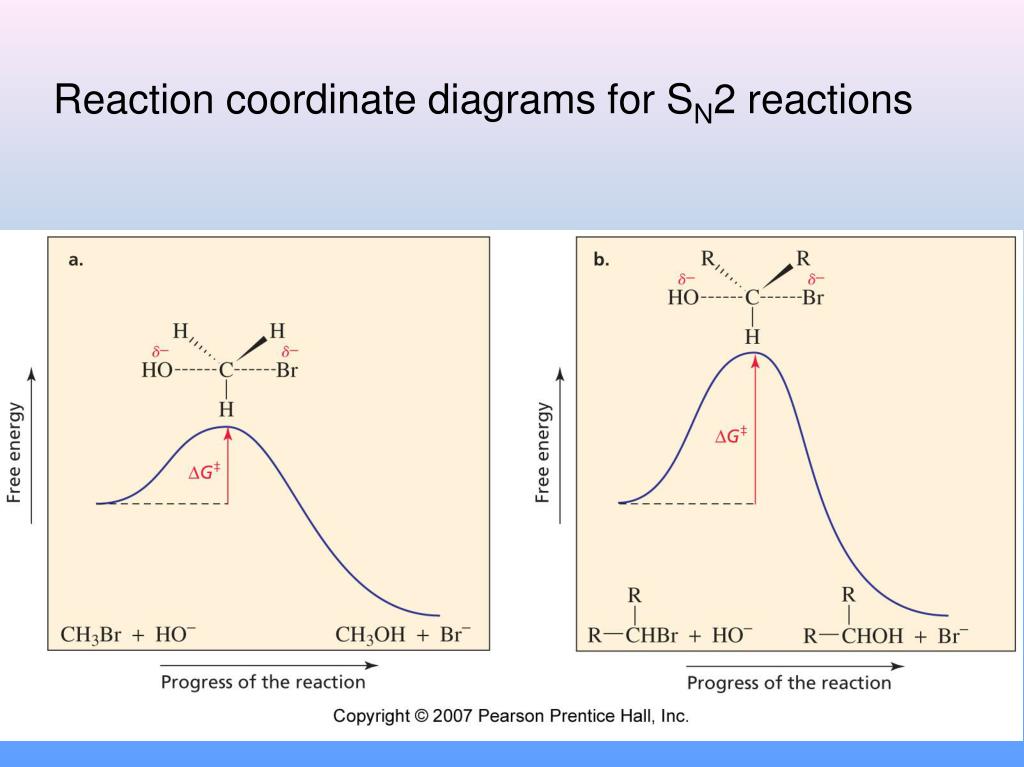
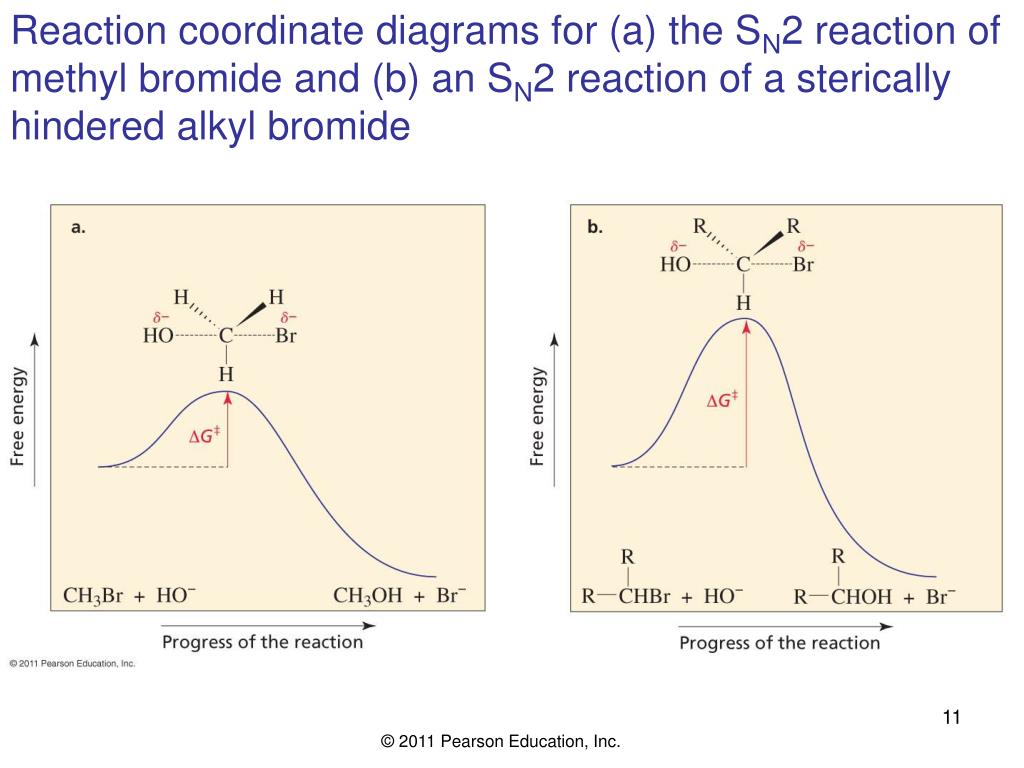
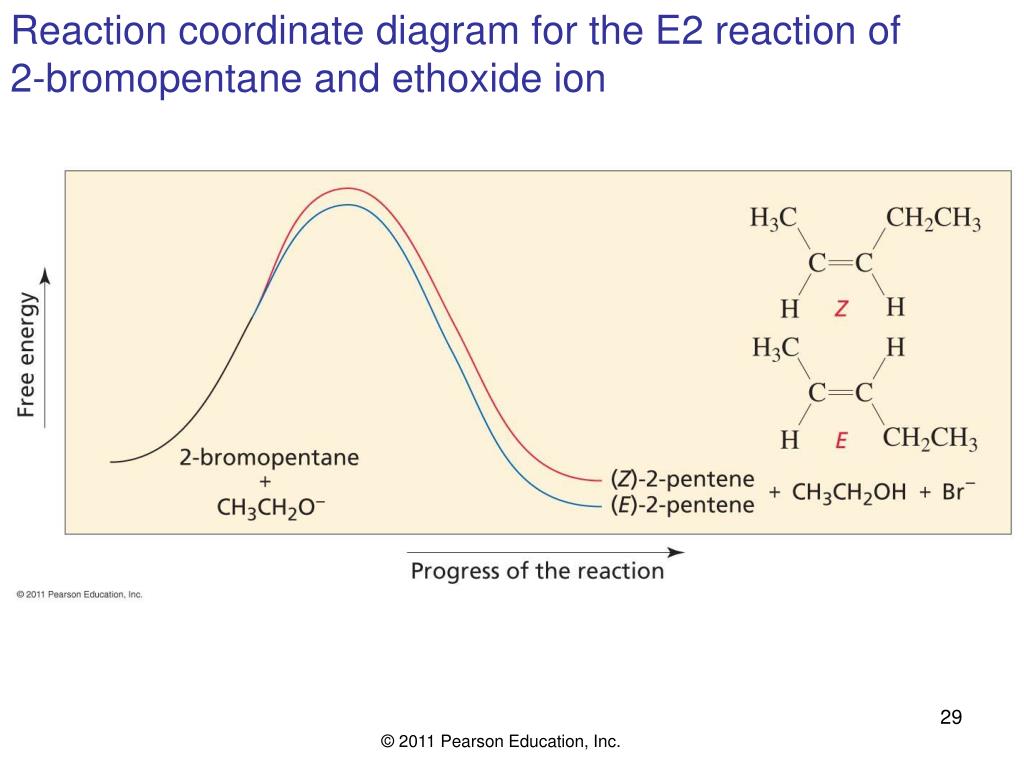
Reaction coordinate diagram Inter and intramolecular examples Solvent trends / solvation S N1 Mechanism / solvolysis Stereochemistry Transition state Reaction coordinate diagram Solvent trends E1 and E2 Mechanisms Stereochemistry Solvent/reaction condition trends Alcohol Synthesis Nucleophilic additions to carbonyls NaH vs. NaBH 4 / LiAlH 4 The reaction coordinate vs. energy diagram has two energy barriers. The bond to the leaving group breaks before the π bond is formed. Select all statements that correctly describe a dehydrohalogenation reaction that proceeds via an E1 mechanism. Reaction coordinate diagram for the E1 reaction of 2-chloro-2-methylbutane. Must consider possible carbocation rearrangement. Stereochemistry of the E1 Reaction. E1 Elimination from Cyclic Compounds E1 mechanism involves both syn and anti elimination. Summary & Applications (Synthesis) S N 1 / E1 vs. S N
E1 reaction coordinate diagram. 2 Reaction Substitution: this reaction involves a substitution of players - two reactants produce two products, in which some things have been switched around: AB + C AC + B Tip: think of this if you get elimination (E1 and E2) reactions mixed up with substitution (SN1 and SN2) reactions. Sn1 Reaction Coordinate Diagram. SN1 reaction is a two step reaction as mentioned below: 1. Leaving group leaves first being solvolysed by solvent creating a carbocation intermediate. This is. whose proposed mechanism and free energy diagram are depicted Figures 1 and 2. Figure 2: Reaction coordinate diagram for an SN1 reaction1. 1. 5.3. Reaction coordinate diagrams. You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams. In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ' reaction coordinate ', tracing from left to right the progress of ... S N 2 mechanism. S N 2 indicates a substitution, nucleophilic, bimolecular reaction, described by the expression rate = k [Nu][R-LG]. This implies that the rate determining step involves an interaction between two species, the nucleophile and the organic substrate. This pathway is a concerted process (single step) as shown by the following reaction coordinate diagrams, where there is ...
Considering the S N 1, S N 2, E1 and E2 mechanisms, the energy diagram shown below corresponds to. a) only the S N 1 mechanism. b) only the S N 2 mechanism. c) both the S N 1 and E1 mechanism. d) both the S N 2 and E2 mechanism. Learn this topic by watching SN1 SN2 E1 E2 Chart (Big Daddy Flowchart) Concept Videos. The E1 mechanism is nearly identical to the S N 1 mechanism, differing only in the course of reaction taken by the carbocation intermediate. As shown by the following equations, a carbocation bearing beta-hydrogens may function either as a Lewis acid (electrophile), as it does in the S N 1 reaction, or a Brønsted acid, as in the E1 reaction. The Energy Diagram for a S N 1 Reaction therefore has an Intermediate! - And is a two step reaction! Potential energy! Reaction Coordinate! I H3C CH3 CH3 OCH3 H3C CH3 CH3 H3C CH3 CH3. Why does a S N 1 Mechanism Occur?! - Whenever the potential energy of a reaction is disfavored for a S N ... E1: Elimination, Unimolecular! This mechanism is ... reaction coordinate E a E a R3CBr R3C reaction coordinate gy R2CH E E a a R2CHBr reaction coordinate gy. 3 ... E1 which competes with S N 1 E2 which competes with S N 2 IV. E2 mechanism (Bimolecular Elimination) ... Energy diagram: B. The Effect of the Base
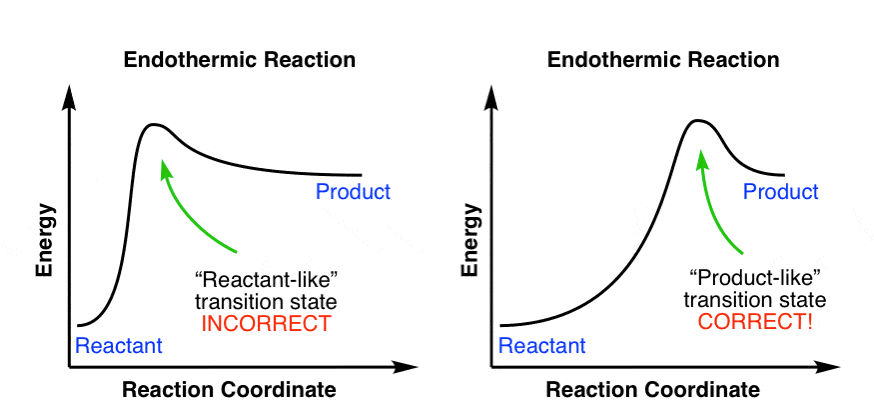
Reaction Coordinate Diagram of Ozone Photolysis The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction . Together, the products O 2 and atomic O, have a higher energy than the reactant O 3 and energy must be added to the system for this reaction. SN1 reaction The S1 reaction is a substitution reaction in organic chemistry. or process an energy profile (or reaction coordinate diagram) is a theoretical. SN1 reaction is a two step reaction as mentioned below: 1. Leaving group leaves first being solvolysed by solvent creating a carbocation intermediate. This is. This reaction is good but check out our new improved animation of the E1 reaction!The E1 reaction with an alkyl halide occurs in two steps. In the first ste... E1 Reactions. Unimolecular elimination (E1) is a reaction in which the removal of an HX substituent results in the formation of a double bond. It is similar to a unimolecular nucleophilic substitution reaction (SN1) in particular because the rate determining step involves heterolysis (losing the leaving group) to form a carbocation intermediate. Because the rate determining (slow) step involves only one reactant, the reaction is unimolecular with a first order rate law.
1! Energy/Reaction Coordinate! Diagrams! Thermodynamics, Kinetics ! Dr. Ron Rusay" A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change (ΔGo) Enthalphy (ΔHo): the heat given off or absorbed during a reaction
Reaction Coordinate! S N 1! Potential energy! Reaction Coordinate! E1! S N 1 and E1 Reactions Have Identical Energy Diagrams for Rate Determining Step! Cl H CH3 3C H3C Cl H CH3 3C H3C CH3 H3C CH3 CH3 H3C CH3 OCH3 H CH3 3C H3C H2C CH3 CH3 B CH3OH Rate = k [substrate]! Rate = k [substrate]!
What substitution reaction mechanism is most likely for the conversion shown? Ym Br; Question: Based upon the energy diagram shown, is this reaction an E1 or an E2 elimination? free energy, kJ/mol reaction coordinate O E1 elimination O E2 elimination O It is impossible to determine from the diagram alone. The diagram suggests that it is not an ...
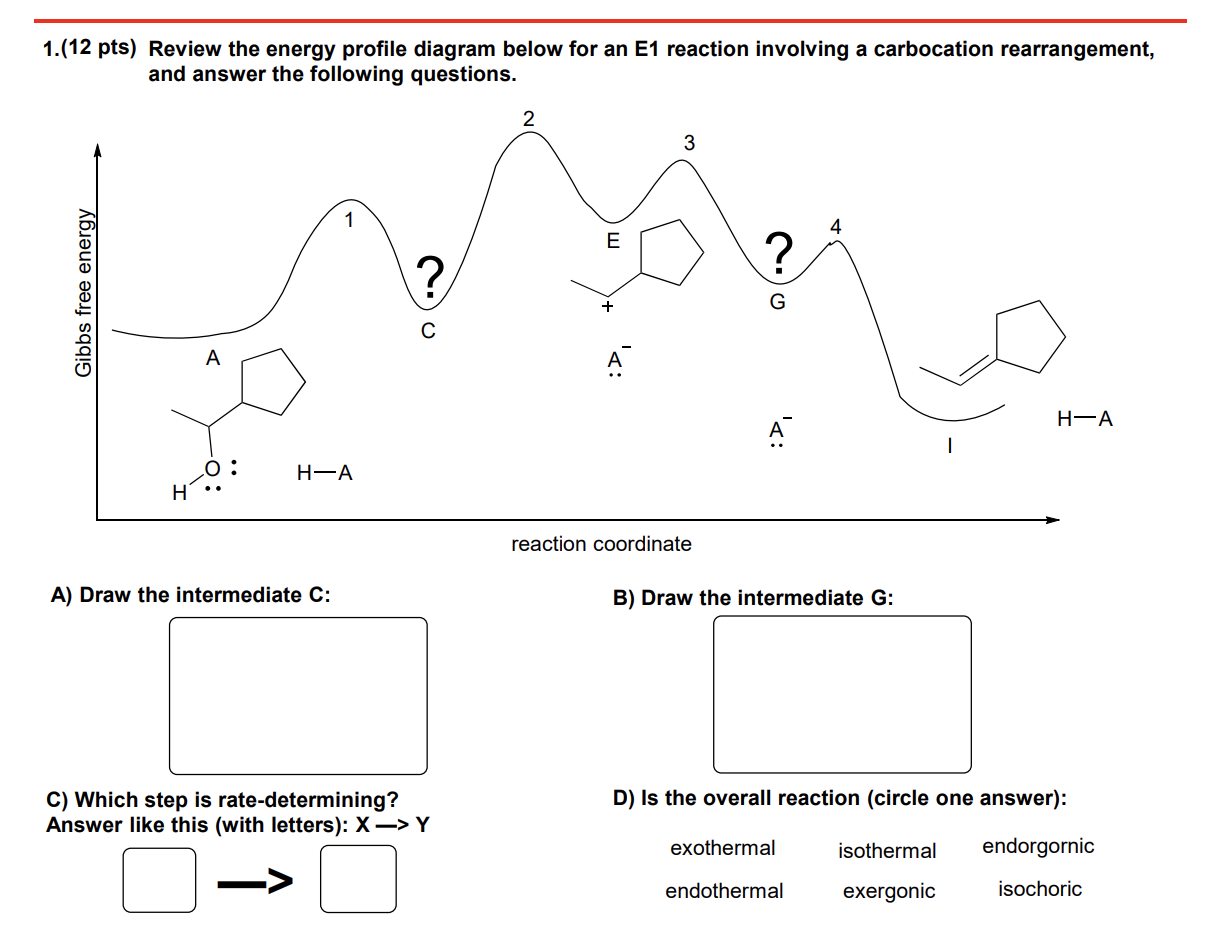
What are characteristics within a reaction coordinate diagram for an E1 reaction?-endothermic -late TS-looks more like the carbocation intermediate -2nd hill = forms the pi bond (PDS) PDS. product determining step -2nd step = controls product distribution.
Transcribed image text: A reaction coordinate diagram is shown below for the reaction of A to form E. Answer the following question. Identify the transition state(s ...
reaction if the reactant is not charged. Ex: H2O, CH3OH, etc. Racemization (with some inversion because of ion pairing) E1 3>2>1 Forms a carbocation Weak base favors E1 reaction by disfavoring E2 reaction Not effected but a low concentration of base favors E1 by disfavoring a E2 reaction Protic polar favors a E1 reaction if the reactant is not ...
SN2 reaction coordinate diagram. In this diagram, there are really only three parts: the reagents, the transition state, and the products. The transition state is the point in the reaction with the highest energy level, and the difference in energy between the reagents and transition state is called the activation energy (often abbreviated as Ea).
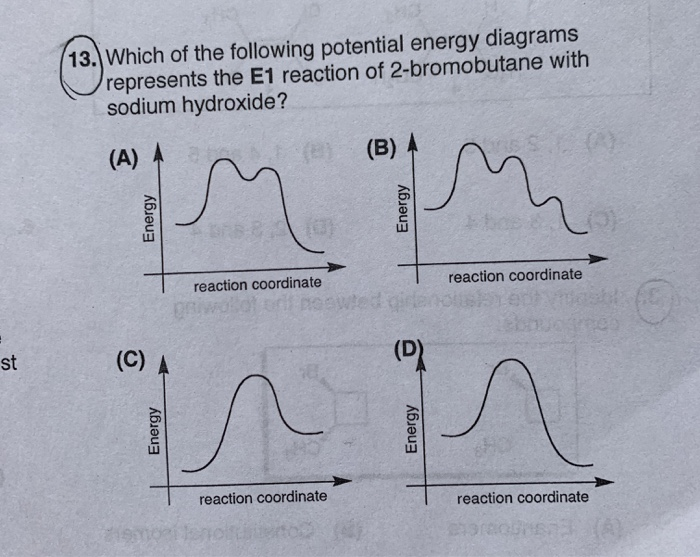
Elimination Reactions and Alkene Synthesis 1) One of the products that results when 1-bromo-2,2-dimethylcyclopentane is heated in ethanol is shown below. Give a mechanism by which it is formed and give the name of this mechanism. CH3 CH3 2) Provide the structure of the major organic product in the following reaction. CH3 H Br D NaOCH3 CH3OH

Draw A Reaction Profile Diagram Of Energy Vs Reaction Coordinate For A Hypothetical Reaction Ab C Arrow A Bc In Which The Foward Reaction Has An Activation Energy Of 100
reaction coordinate Br Figure 9.11 Reaction free-energy diagram for the S N1–E1 solvolysis reaction of (CH 3) 3CBr with ethanol.The rate-limiting step,ionization of the alkyl halide (red curve),has the transition state of highest standard free energy.The
https://Leah4sci.com/elimination presents: E1 Reaction Coordinate Energy Diagram with step by step mechanism, transition states and intermediates📺Watch Next...
reaction coordinate (E2) energy ‡ X H R R R R δ B − δ− ‡ R X R R R R R R R B H B – δ− δ+ ‡ reaction coordinate (E1) energy ‡ RDS ‡ R X R R R B – R R R R B H R R R R B – X – H R R R R B CHR 2 X R R δ− δ+ Bimolecular Unimolecular Substitution Elimination Generic Reaction-Energy Diagrams Predicting the Products: Substitution versus Elimination Is Nuc/Base strong? no Unimolecular Reaction Bimolecular yes Reaction
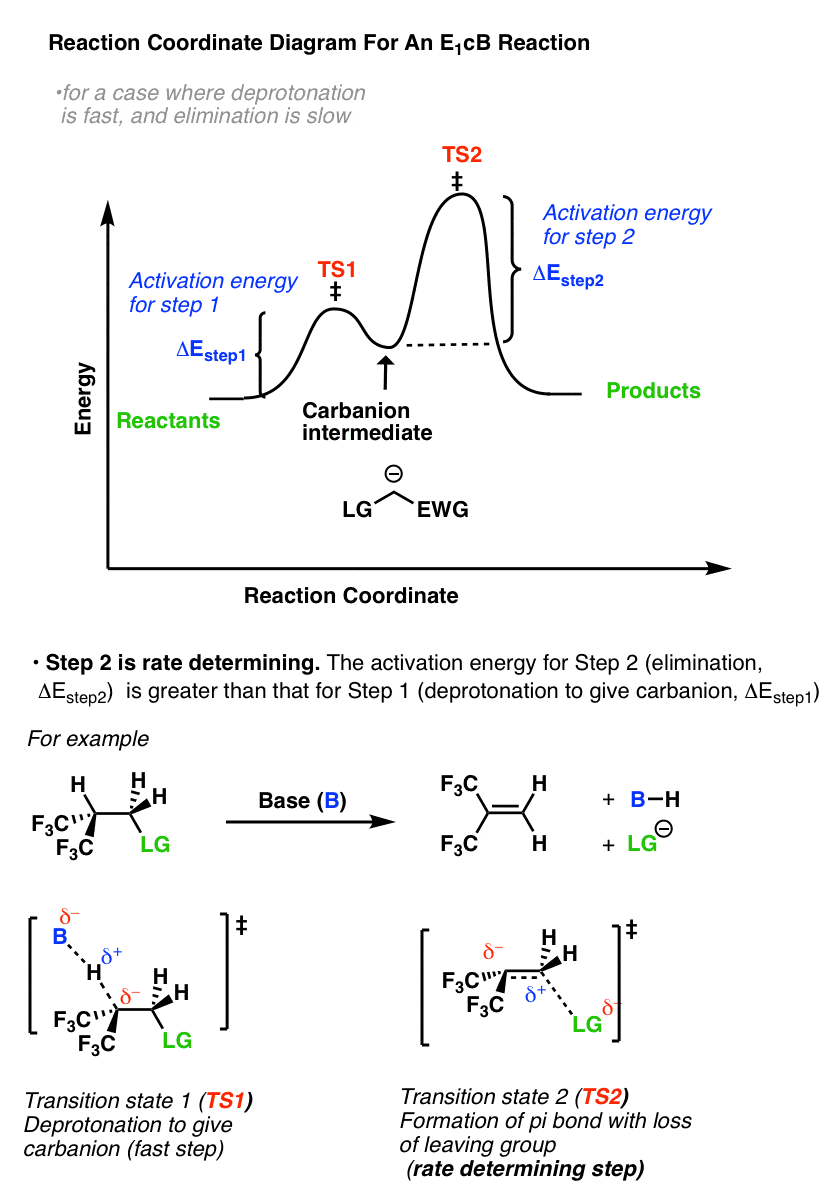
Let’s break down the steps of the E1 reaction and characterize them on the energy diagram: Step 1: Loss of he leaving group. The energy diagram of the E1 mechanism demonstrates the loss of the leaving group as the slow step with the higher activation energy barrier: The dotted lines in the transition state indicate a partially broken C-Br bond. The Br being the more electronegative element is partially negatively charged and the carbon is partially positively charged. Complete ionization of the bond leads to the formation of the carbocation intermediate. Step 2: Removing a β-hydrogen to form a π bond. Once the carbocation is formed, it is quickly attacked by the base to remove the β-hydrogen forming an alkene. Notice the smaller activation energy for this step indicating a faster reaction: In the next section, we will discuss the features of SN1 and E1 reactions as well as strategies to favor elimination over substitution.

Organic Chemistry Students Challenges With Coherence Formation Between Reactions And Reaction Coordinate Diagrams Chemistry Education Research And Practice Rsc Publishing Doi 10 1039 C8rp00064f
Reaction coordinate diagram for the E1 reaction of 2-chloro-2-methylbutane. Must consider possible carbocation rearrangement. Stereochemistry of the E1 Reaction. E1 Elimination from Cyclic Compounds E1 mechanism involves both syn and anti elimination. Summary & Applications (Synthesis) S N 1 / E1 vs. S N
The reaction coordinate vs. energy diagram has two energy barriers. The bond to the leaving group breaks before the π bond is formed. Select all statements that correctly describe a dehydrohalogenation reaction that proceeds via an E1 mechanism.
Reaction coordinate diagram Inter and intramolecular examples Solvent trends / solvation S N1 Mechanism / solvolysis Stereochemistry Transition state Reaction coordinate diagram Solvent trends E1 and E2 Mechanisms Stereochemistry Solvent/reaction condition trends Alcohol Synthesis Nucleophilic additions to carbonyls NaH vs. NaBH 4 / LiAlH 4
















0 Response to "38 e1 reaction coordinate diagram"
Post a Comment