37 match the appropriate octahedral crystal-field splitting diagram
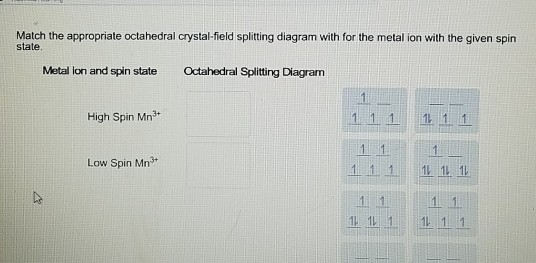
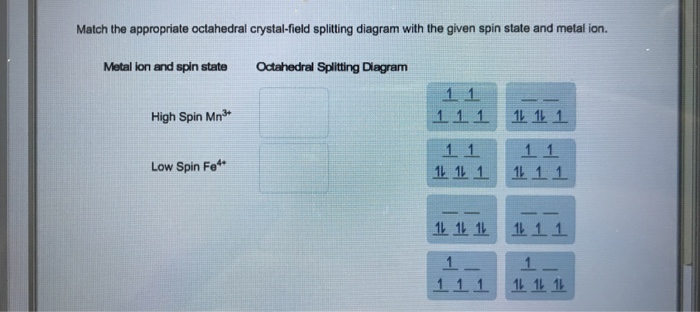
Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. High-spin Mn^3+ Low-spin Fe^3+. Sign up to view answer. Our mission is to help you succeed in your Chemistry class. Sign up for free to see the solution. Thus it is clear that t 2 g orbitals are nearer to the ligands than the eg orbitals. Hence t2g orbitals will experience more repulsion than eg orbitals. Therefore, crystal field splitting will be reversed of octahedral field which can be shown as below.
Question match the appropriate octahedral crystal field splitting diagram with the given spin state and metal ion. A left handed propeller will pull the stern to starboard right when in reverse. Given this is an octahedral complex your splitting diagram will have 2 degenerate states. Crystal field splitting in an octahedral field eg energy 35 o ...
Match the appropriate octahedral crystal-field splitting diagram
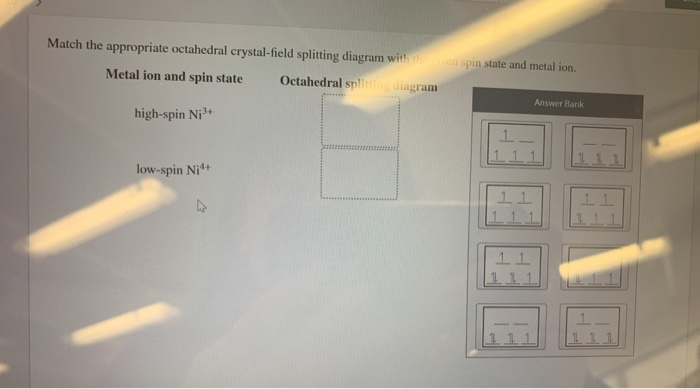
Chemistry questions and answers. Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral splitting diagram Answer Bank high-spin Ni3+ low-spin Fe4+. Match the appropriate octahedral crystal field splitting diagram. As a result the splitting observed in a tetrahedral crystal field is the opposite of the splitting in an octahedral complex. The octahedral ion feno 2 6 3 which has 5 d electrons would have the octahedral splitting diagram shown at right with all five electrons in the t 2g level. Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Weekly leaderboard. Home. Homework Help 3,800,000. Chemistry 820,000.
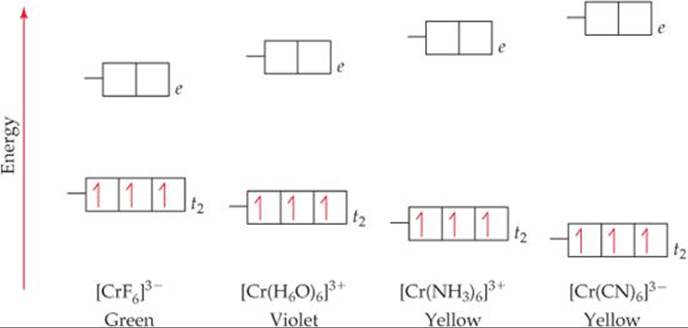
Match the appropriate octahedral crystal-field splitting diagram. This photo about: Match the Appropriate Octahedral Crystal-field Splitting Diagram, entitled as Ligand Field Theory Advanced Inorganic Chemistry Lecture Slides Match The Appropriate Octahedral Crystal Field Splitting Diagram - also describes Ligand Field Theory Advanced Inorganic Chemistry Lecture Slides and labeled as: match the following phrases about colour,match the phrases,match the ... Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral Splitting Diagram High Spin Fe^3+ Low Spin Co^2+ Crystal Field Splitting in an Octahedral Field eg Energy 3/5 o o 2/5 o t2g e g - The higher energy set of orbitals (d z2 and d x2-y2) t 2g - The lower energy set of orbitals (d xy, d yz and d xz) Δ o or 10 Dq - The energy separation between the two levels The eThe eg orbitals are repelled by an amount of 0 6orbitals are repelled by an amount of 0.6 Δo The t2gorbitals to be stabilized to the ... Match the appropriate octahedral crystal field splitting diagram with the given spin state and metal ion. The splitting diagram for square planar complexes is more complex than for octahedral and tetrahedral complexes and is shown below with the relative energies of each orbital. As a result the splitting observed in a tetrahedral crystal field ...
The octahedral crystal field splitting diagram for is as followscthe electron configuration of is. 1 draw the octahedral crystal field splitting diagram for each metal ion. Fe2 low spin is broken down into a number of easy to follow steps and 23 words. Answer to draw the octahedral crystal field splitting diagram for each metal ion. Calculate ... The d-orbital splitting diagram is the inverse of that for an octahedral complex. 6. 7. Page 7 of 33 Crystal Field Splitting Parameters In an octahedral or a tetrahedral crystal field, the d-orbitals are split into two sets. The energy separation between them is called the crystal field splitting parameter. Match the appropriate octahedral crystal field splitting diagram match the appropriate octahedral crystal field splitting diagram with the given spin state and metal ion. Because none of the d orbitals points directly at the ligands in a tetrahedral complex these complexes have smaller values of the crystal field splitting energy δ t. Match the appropriate octahedral crystal field splitting diagram. A none of the 3d orbitals point directly at ligands b t2 orbitals are more stable than e orbitals c a small crystal field splitting energy results in a paramagnetic complex d the low spin case gives maximum unpaired electrons e for a given ligand.
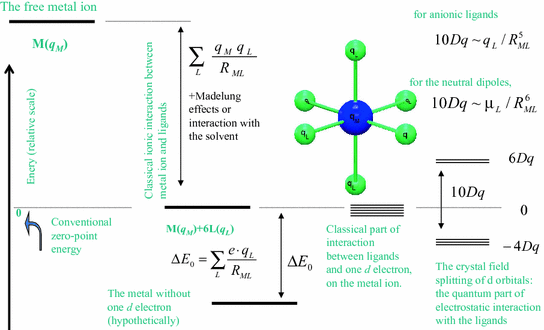
Answer to Construct the octahedral crystal-field splitting diagram for the metal in each species. V (H2O)63+ Co (CN)63 - Mn (H2O)62+. A d1 octahedral complex is found to absorb visible light, with the absorption maximum occcurring at nm. a) Calculate the crystal-field splitting energy, Δ, in. Crystal field theory was established in 1929 treats the interaction of metal ion and ligand as a purely electrostatic phenomenon where the ligands are considered as point charges in the vicinity of the atomic orbitals of the central atom. Development and extension of crystal field theory taken into account the partly covalent nature of bonds ... Crystal field splitting diagram for octahedral posted on april 4 2019 by admin image for match the appropriate octahedral crystal field splitting diagram with given spin state the following is a crude and approximate molecular orbital diagram for an octahedral complex octahedral crystal field stabilization energy octahedral splitting with 2. Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral splitting diagram Answer Bank high-spin Mn2+ 1 1 1 1 1 1 1 1 1. 11 11 11 low-spin Mn2+ 1L 1L 1 1 1 1 1 1 1 11 1 1 1 1 1
CRYSTAL-FIELD SPLITTING DIAGRAMS All four of these transition metals commonly have coordination numbers of \mathbf(6), however, so let's examine their octahedral complex crystal-field splitting diagrams. HIGH SPIN VS. LOW SPIN High spin = fill all five d orbitals with one electron first, and then double up. Low spin = fill lowest-energy d ...
Construct the octahedral crystal field splitting diagram. Called crystal field theory. If the splitting of the d orbitals in an octahedral field is δoct the three t2g orbitals are stabilized relative to the barycenter by 25 δoct and the eg orbitals are destabilized by 35 δoct. Basically the question is referring to the compound k3fec2o43.
A d1 octahedral complex is found to absorb visible light with the absorption maximum occcurring at 523 nm. Match the appropriate octahedral crystal field splitting diagram fe4. Crystal field splitting in an octahedral field eg energy 35 o o 25 o t2g e g the higher energy set of orbitals d z2 and d x2 y2 t 2g the lower energy set of orbitals d ...

Easy Plane To Easy Axis Anisotropy Switching In A Co Ii Single Ion Magnet Triggered By The Diamagnetic Lattice Journal Of Materials Chemistry C Rsc Publishing
Knowing that halides are weak-field ligands, the maximum number of unpaired spins (i.e., high spin) is expected in this octahedral complex. That is, in an octahedral-splitting diagram, the three t2g orbitals and two eg orbitals will be singly occupied before any t2g orbitals are completely filled.
Answer to: Match the appropriate octahedral crystal-field splitting diagram with the given spin stale and metal ion with given spin state. [{Image...
Crystal Field Splitting Diagram For Octahedral Written By JupiterZ Sunday, February 21, 2021 Add Comment Edit Http Iopscience Iop Org Article 10 1088 1742 6596 667 1 012008 Pdf
Question match the appropriate octahedral crystal field splitting diagram with the given spin state and metal ion. Match the appropriate octahedral crystal field splitting diagram. The octahedral ion feno 2 6 3 which has 5 d electrons would have the octahedral splitting diagram shown at right with all five electrons in the t 2g level.
The difference between the energies of the t 2g and e g orbitals in an octahedral complex is represented by the symbol o.This splitting of the energy of the d orbitals is not trivial; o for the Ti(H 2 O) 6 3+ ion, for example, is 242 kJ/mol. . The magnitude of the splitting of the t 2g and e g orbitals changes from one octahedral complex to another. It depends on the identity of the metal ion ...

Match The Appropriate Octahedral Crystal Field Splitting Diagram With The Given State And Metal Ion Metal Ion Homeworklib
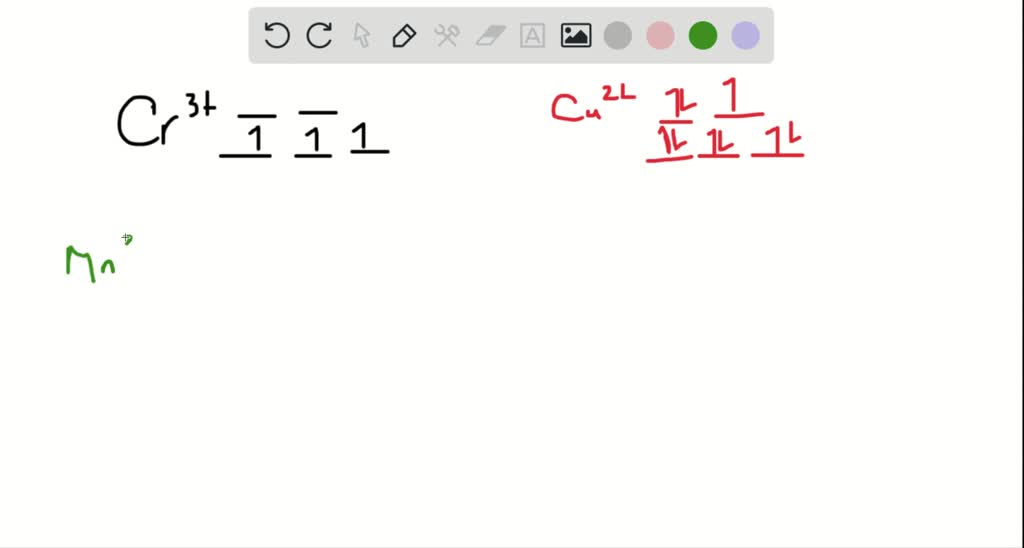
Explanation:- The valance electron configuration of Fe is 3d⁶4s². So the electron configuration for Fe3+ should be 3d⁵ …. View the full answer. Transcribed image text: Match the appropriate octahedral crystal-field splitting diagram with for the metal ion with the given spin state.
Construct the octahedral crystal field splitting diagram for the metal in each species. Since the oxalate ligand is fairly low in the series a weak field ligand at this point you may not have studied ligand field theory yet which explains why it is a weak ligand. Cr4 mnh2o62 asked by katie on march 30 2012 chemistry based on crystal field ...

A Theoretical Perspective On Charge Separation And Transfer In Metal Oxide Photocatalysts For Water Splitting Zhou 2019 Chemcatchem Wiley Online Library
question match the appropriate octahedral crystal field question match the appropriate octahedral crystal field splitting diagram with the given spin state and metal ion high spin cr 2 low spin fe 3 please give the octahedral splitting diagrams for each ion. crystal field stabilization energy Calculation of the LFSE of tetrachloridocobaltate II.
Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Weekly leaderboard. Home. Homework Help 3,800,000. Chemistry 820,000.
Match the appropriate octahedral crystal field splitting diagram. As a result the splitting observed in a tetrahedral crystal field is the opposite of the splitting in an octahedral complex. The octahedral ion feno 2 6 3 which has 5 d electrons would have the octahedral splitting diagram shown at right with all five electrons in the t 2g level.
Chemistry questions and answers. Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral splitting diagram Answer Bank high-spin Ni3+ low-spin Fe4+.

Chinese Original Book With No Abridgment The Art Of War Chinese The Most Classic Literature Hardcover Version For Collection Art Sketch Book Art Stonearts And Crafts Bathroom Aliexpress
Solved Construct The Octahedral Crystal Field Splitting Diagram For The Metal In Each Species Course Hero
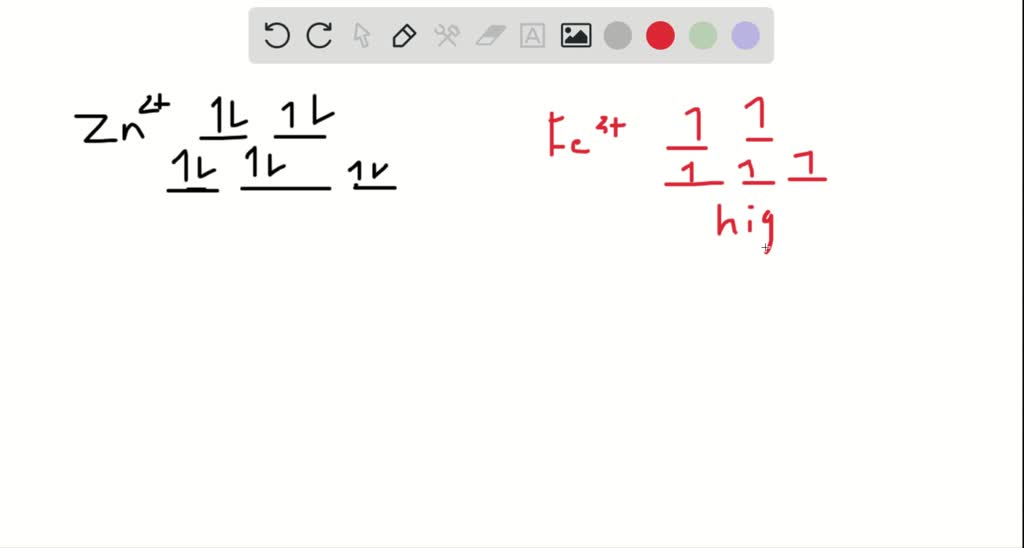
Solved Draw The Octahedral Crystal Field Splitting Diagram For Each Metal Ion Begin Array Ll Text A Z N 2 Text B F E 3 Text High And Low Spin Text

Help With This Question Please Match The Appropriate Octahedral Crystal Field Splitting Diagram With The Given Spin Homeworklib
Frontiers Practical And Computational Studies Of Bivalence Metal Complexes Of Sulfaclozine And Biological Studies Chemistry
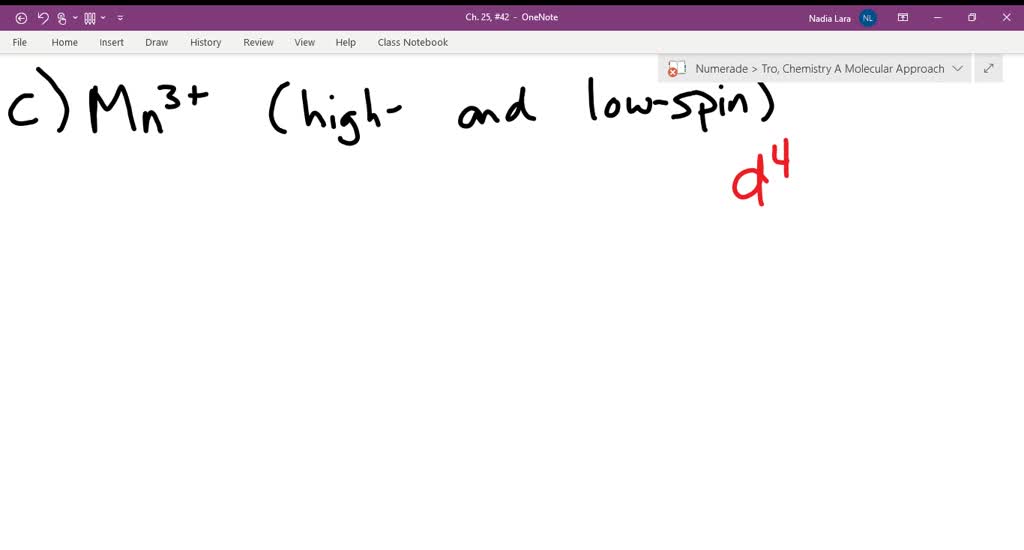
Solved Match The Appropriate Octahedral Crystal Field Splitting Diagram With For The Metal Ion With The Given Spin State Metal Ion And Spin State Octahedral Splitting Diagram Answer Bank High Spin Fe 11 11












0 Response to "37 match the appropriate octahedral crystal-field splitting diagram"
Post a Comment