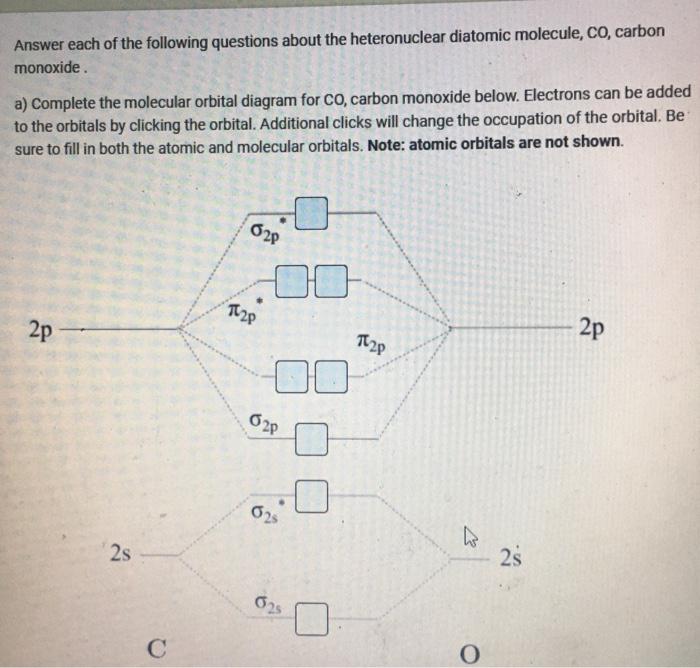
40 heteronuclear molecular orbital diagram
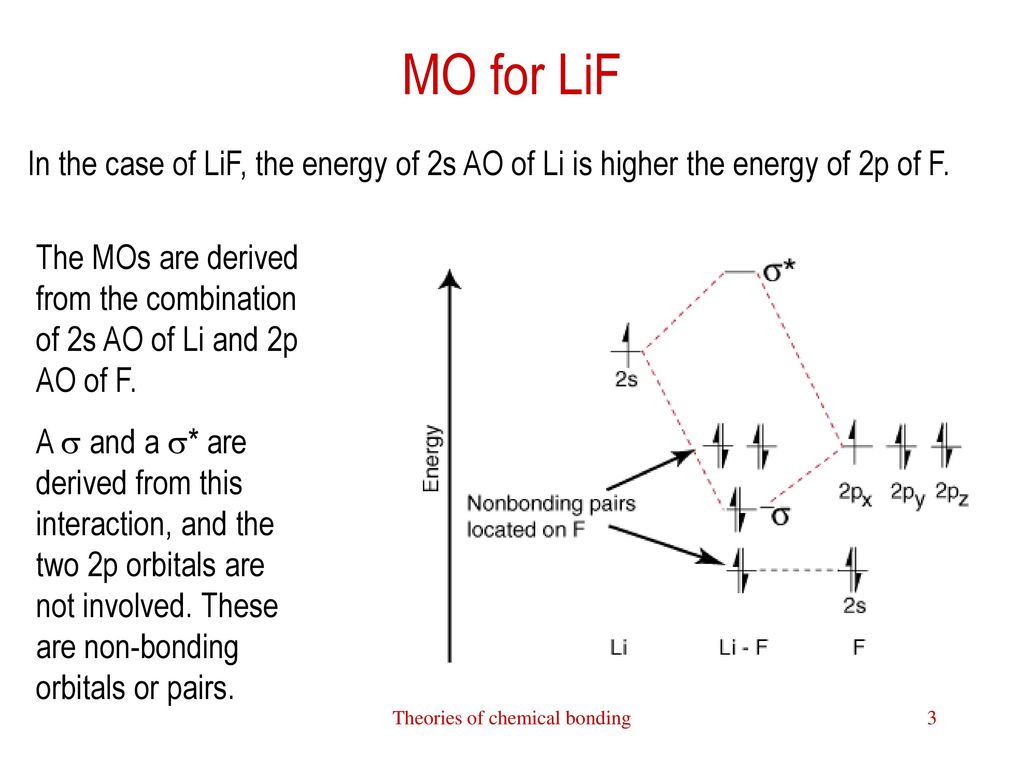
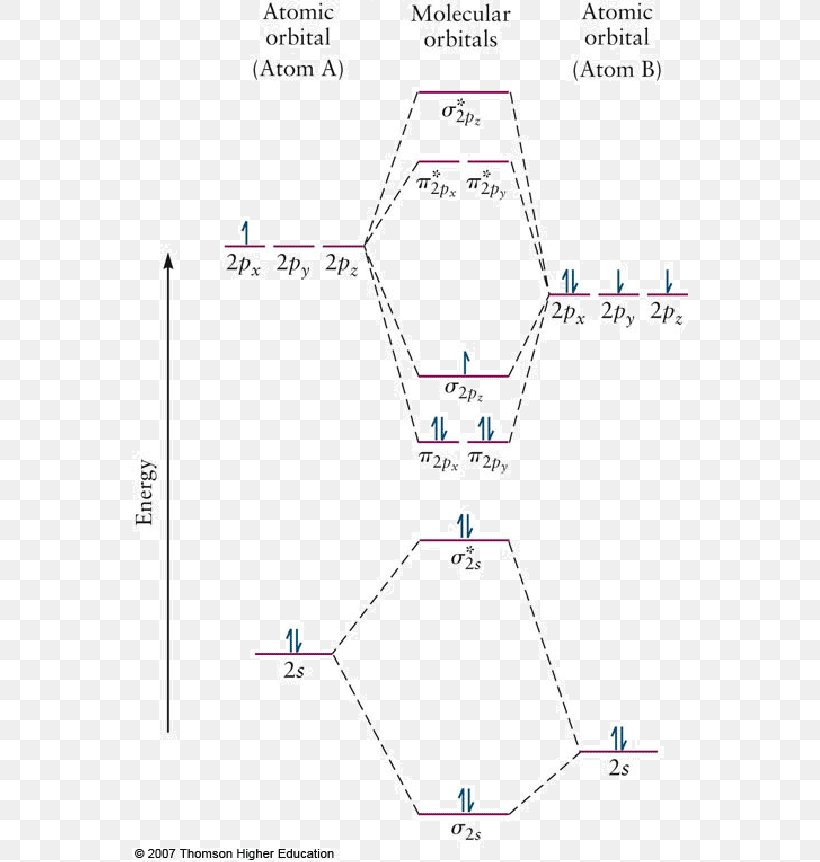
Asked for: "skewed" molecular orbital energy-level diagram, bonding... : Molecular Orbital Energy-Level Diagram for a Heteronuclear Diatomic Molecule AB, Where χ B > χ A . The bonding molecular orbitals are closer in energy to the atomic orbitals of the more electronegative B atom. Consequently, the electrons in the bonding orbitals are not shared equally... Molecular Orbital Theory Heteronuclear Diatomic (Cyanide, CN...) Dr. Shields shows you how to draw the MO correlation diagram for cyanide (CN-), calculate the MO bond order, and write the MO electron configuration with an...
Molecular Orbitals: Molecular Orbital Theory | SparkNotes Figure %: Orbital correlation diagram for homonuclear diatomic molecules other than B2, C2, and N2. To draw the correlation diagrams for heteronuclear diatomic molecules, we face a new problem: where do we place the atomic orbitals on an atom relative to atomic orbitals on other atoms?
Heteronuclear molecular orbital diagram
MO Diagrams | Molecular Orbital Diagram Maker A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. p-Block Heteronuclear diatomic molecules: Main Group Polyatomic Molecules. PDF set2b.ppt | Heteronuclear Molecules Heteronuclear Molecules. 2-43. • The relative energy of the bonding orbitals determines the • As the polarity difference between two atoms increases, the orbital energy difference also increases • Many properties of molecules can be interpreted through the use of the molecular orbital model, and in... Molecular orbitals of heteronuclear diatomic molecules For drawing MO diagram for heteronuclear diatomic molecule, how do I know which is lower in energy than the other? Involving heavier atoms makes it harder to guess at molecular orbital diagrams, and there is need for quantum chemistry calculations.
Heteronuclear molecular orbital diagram. PDF Microsoft PowerPoint - Symmetry-and-Molecular-Orbitals.htm Molecular Orbital Energy Level Diagram. •Better Overlap => Higher DE •Bond Order = ½(# of B.O. e - - # of A.O. e -) •Diamagnetic: all e- paired •Paramagnetic: with e- unpaired. Heteronuclear Diatomic Molecules. • A more electronegative • B less electronegative. Molecular Orbital Diagrams simplified | by Megan A. Lim | Medium Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular… Heteronuclear Diatomic Molecules. Because the electronegativity of the two atoms are unequal, the... Molecular orbital | Detailed Pedia | Heteronuclear diatomics Molecular orbital (Redirected from Molecular Orbital). Wave-like behavior of an electron in a molecule. The general procedure for constructing a molecular orbital diagram for a reasonably simple molecule can be summarized as follows Molecular Orbitals - Molecular Orbitals for Heteronuclear Molecules The molecular orbitals in the heteronuclear case will in general be concentrated more around one nucleus than the other. The molecular orbital is said to be approximated mathematically by a linear combination of atomic orbitals and the technique is known as the LCAO-MO method.
Tutorial on Chemical Bonding, Part 8 of 10 (Molecular orbitals) The molecular orbital model is by far the most productive of the various models of chemical bonding, and serves as the basis for most quantiative calculations The resulting molecule is 243 kJ/mole more stable than the parent atoms. As we might expect, the bond energy of the heteronuclear molecule is... 8.4 Molecular Orbital Theory - Chemistry Figure 8. This is the molecular orbital diagram for the homonuclear diatomic Be2+, showing the molecular orbitals of the valence shell only. The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. Molecular Orbital Theory Diatomic molecules: Heteronuclear... 3 Molecular Orbital Theory MO diagrams for other heteronuclear diatomics are formed in exactly the same way as that of H-F or those of the homonuclear diatomics: atomic orbitals of appropriate symmetry will interact to produce MO's. The orbtals that are closest in energy to one another will... PDF Chapter 5 | 5.2.2 Orbital Mixing The s molecular orbital is a bonding molecular orbital, and has a lower energy than the original atomic orbitals, since this combination of atomic in the schematic sketches on the left of the energy level diagram and in the calculated molecular orbital images on the right.* Because c(1sb) 4 , and.
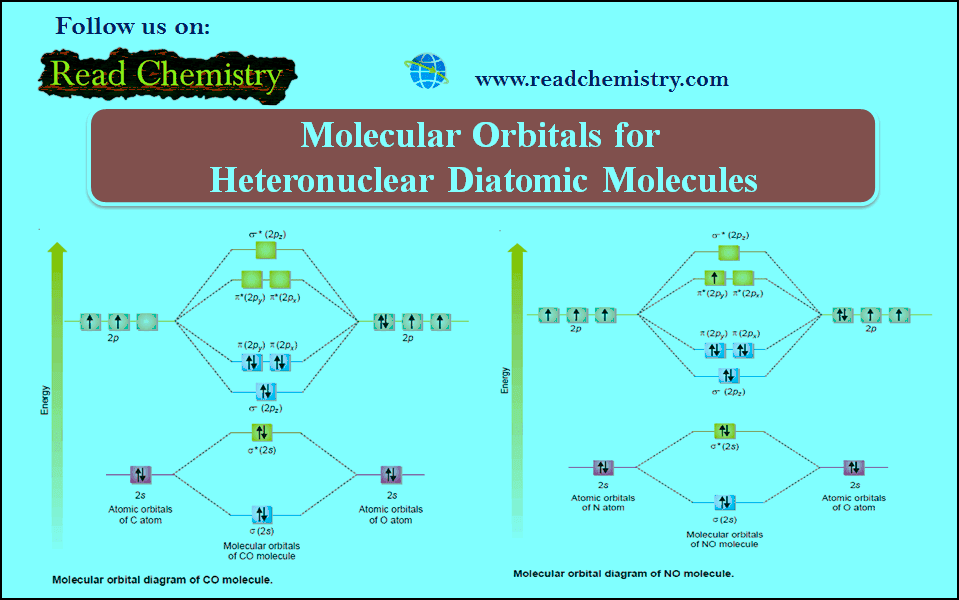
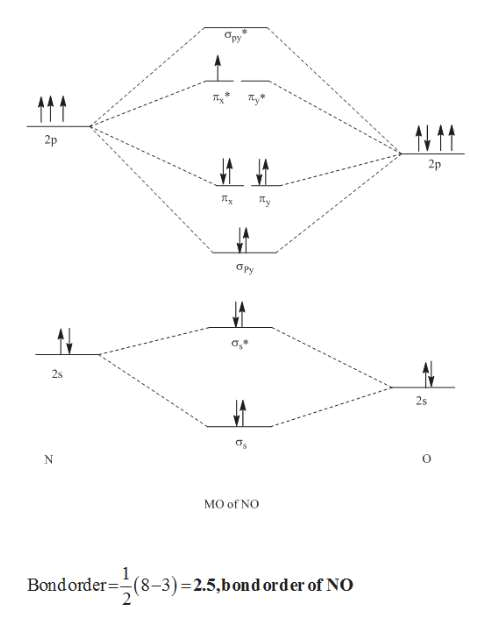
What is the molecular orbital diagram for NO? - Quora You can find the molecular orbital diagram for NO- at the very bottom of this page Form the above molecular orbital electronic configuration of NO molecule , it has been found that the number of bonding electron is 10 and the number of anti bonding electron is 5 . Introduction to Inorganic Chemistry/Molecular Orbital Theory... Valence bond (VB) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons. Molecular orbitals theory Molecular Orbital - A combination of atomic orbitals in molecular orbital theory that provides an orbital description of a molecule analogous to the atomic The construction of other heteronuclear diatomic orbital correlation diagrams follows exactly the same principles as those we employed for LiF. Chapter 6 - Molecular Structure Each molecular orbital is characterized by an energy level, and the electrons in a molecule fill the molecular The two nuclei in heteronuclear diatomic molecules are nuclei of different elements, so the Use the MO diagram in the figure to determine the number of bonding interactions, the number...
Molecular Orbital Theory: Energy level diagram for molecular orbitals Molecular orbital theory was put forward by Hund and Mullikan in 1932. This theory is modern equal energies (in case of homonuclear molecules) or of comparable energies (in case of heteronuclear This energy diagram for the molecular orbitals is shown in Fig.1 However, experimental evidence for...
PDF Molecular Orbitals in | 9-2 Molecular Orbital Energy Level Diagrams Accordingly, a molecular orbital diagram such as Figure 9-5 is inappropriate for heteronuclear diatomic molecules. If the two elements are similar (as in NO or CN mole-cules, for example), we can modify the diagram of Figure 9-5 by skewing it slightly.
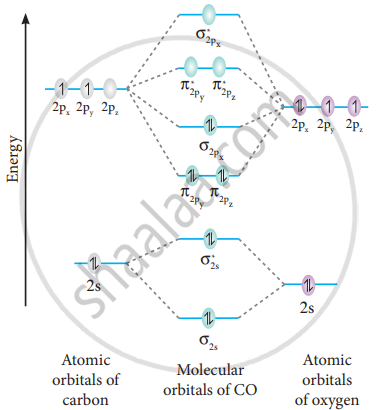
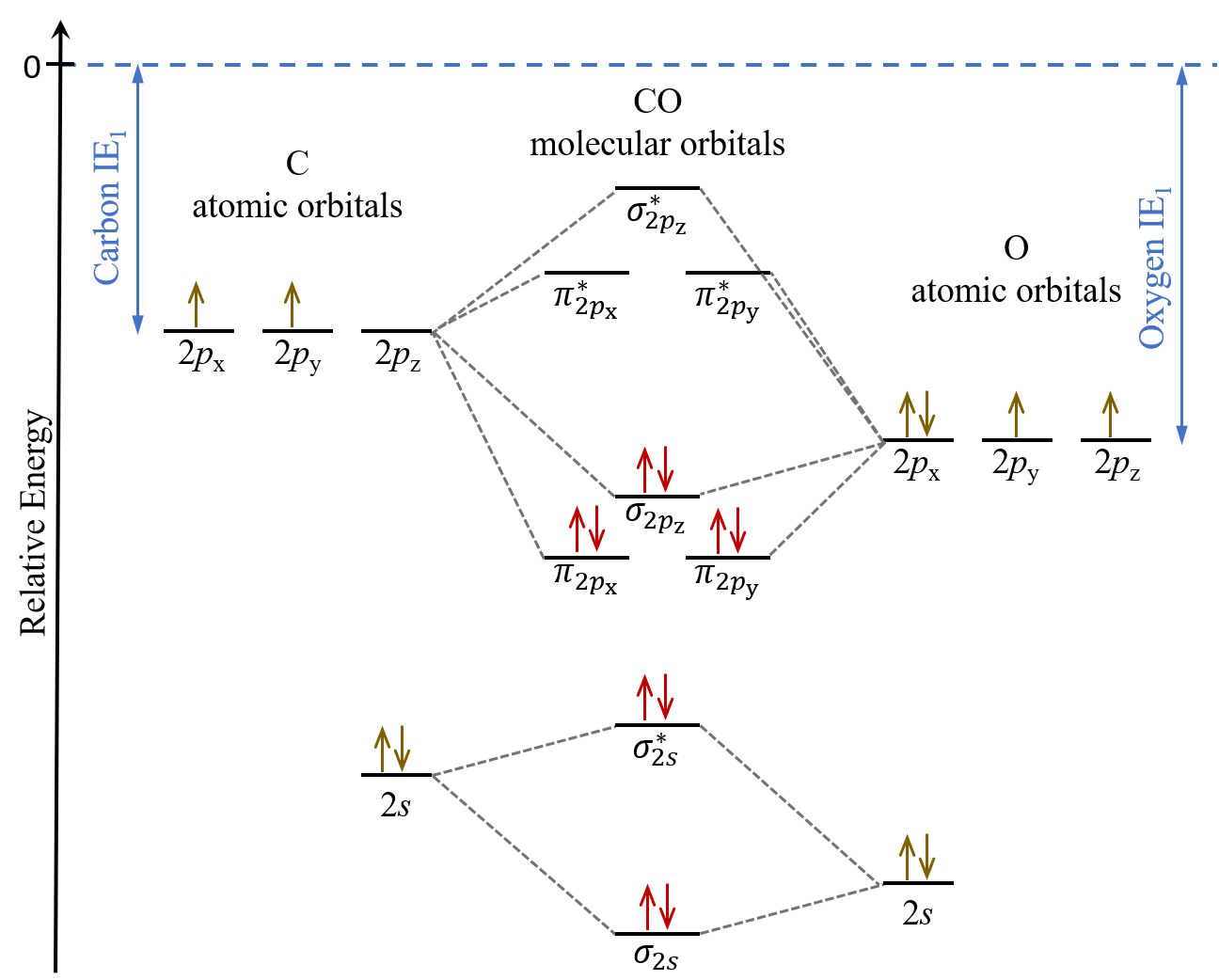
Molecular Orbital Theory - MO diagram of Heteronuclear Diatomic... Molecular Orbital Diagram of heteronuclear diatomic molecules - Comparison of bond order, bond dissociation energy, bond length etc. of different species.
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
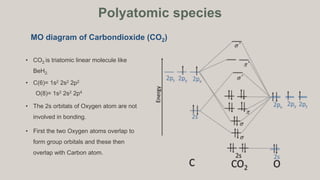
PDF Slide 1 In molecular orbital (MO) approach - overlap orbitals for the whole molecule -bonding is therefore DELOCALISED. We will look first at DIATOMIC MOLECULES and only later move on to POLYATOMIC MOLECULES. Molecular Orbital Energy Level Diagram for a Heteronuclear Diatomic.
PPT - Molecular Orbital Theory PowerPoint Presentation, free... Molecular Orbital Theory Diatomic molecules: Heteronuclear molecules In heteronuclear diatomic molecules, the relative contribution of MO diagram for HF Molecular Orbital Theory Because the contributions are not equal, the MO diagram will be skewed. There is a little bit of mixing between the...
03 Molecular Orbitals of Heteronuclear Diatomics | Susan Findlay Exercise 3.2: Molecular Orbital Diagrams of Heteronuclear Diatomics. 139 KB. 01 Reviewing Atomic Orbitals, Electron Configurations and Lewis Diagrams. 02 Molecular Orbitals of Homonuclear Diatomics.
PDF Molecular Orbitals Molecular Orbital Diagrams. 1. Electrons preferentially occupy molecular orbitals that are lower in For heteronuclear molecules: 1. The bonding orbital(s) will reside predominantly on the atom of lower orbital Group theory is usually used to develop molecular orbital diagrams and drawings of more...
Molecular orbital theory in heteronuclear diatomics | Quizlet Describe how to draw a molecular orbital diagram for a heteronuclear diatomic molecule. -List the occupied atomic orbitals of both atoms, labelling Describe molecular orbital formation in molecule XY, where X and Y each contribute a 2s electron electron to a 2s orbital, drawing a molecular...
"Molecular orbital diagram." In heteronuclear diatomic molecules, atomic orbitals only mix when the electronegativity values are similar. While MOs for homonuclear diatomic molecules contain equal contributions from each interacting atomic orbital, MOs for heteronuclear diatomics contain different atomic orbital...
Molecular orbitals heteronuclear diatomics - Big Chemical... The molecular orbital energy-level diagrams of heteronuclear diatomic molecules are much harder to predict qualitatitvely and we have to calculate each one explicitly because the atomic orbitals contribute differently to each one.
PDF Microsoft PowerPoint - An introduction to Molecular Orbital Theory.ppt... • Molecule orbital theory (Robert Mullikan). • Electrons are delocalised - Different to Lewis and hybridisation (these are not MO). • Energy level diagram represents this interaction. - Two s orbitals interaction to create a low energy bonding and high energy anti-bonding molecular orbital.
Molecular orbitals of heteronuclear diatomic molecules For drawing MO diagram for heteronuclear diatomic molecule, how do I know which is lower in energy than the other? Involving heavier atoms makes it harder to guess at molecular orbital diagrams, and there is need for quantum chemistry calculations.
PDF set2b.ppt | Heteronuclear Molecules Heteronuclear Molecules. 2-43. • The relative energy of the bonding orbitals determines the • As the polarity difference between two atoms increases, the orbital energy difference also increases • Many properties of molecules can be interpreted through the use of the molecular orbital model, and in...
MO Diagrams | Molecular Orbital Diagram Maker A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. p-Block Heteronuclear diatomic molecules: Main Group Polyatomic Molecules.









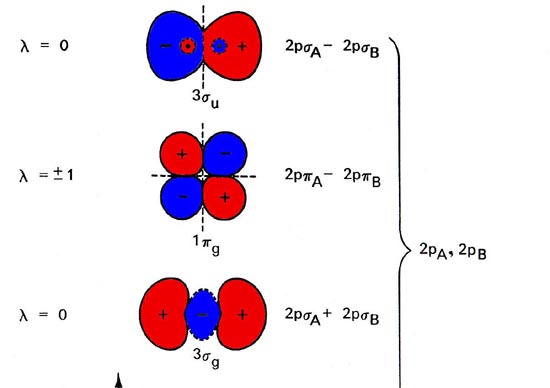
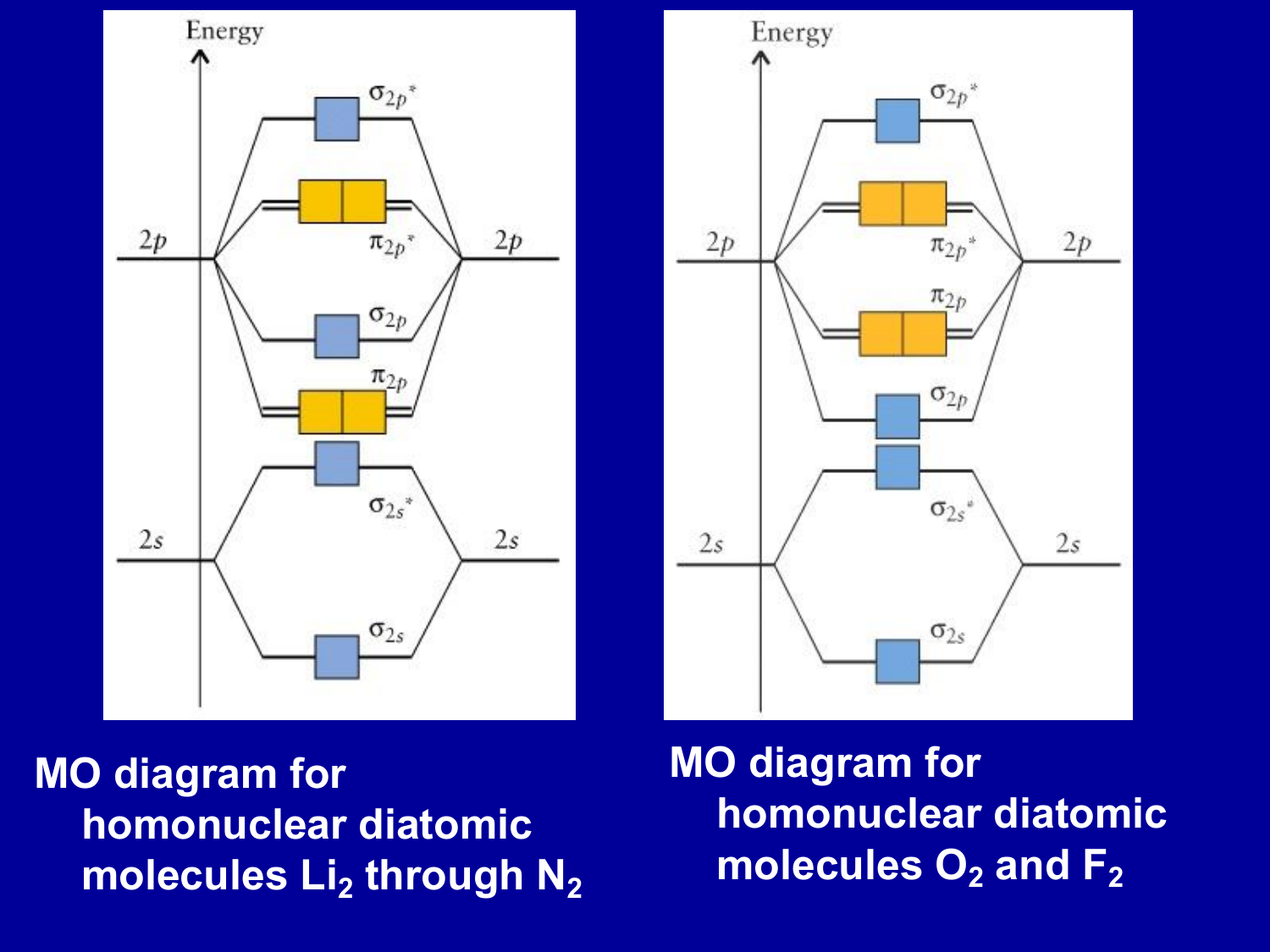



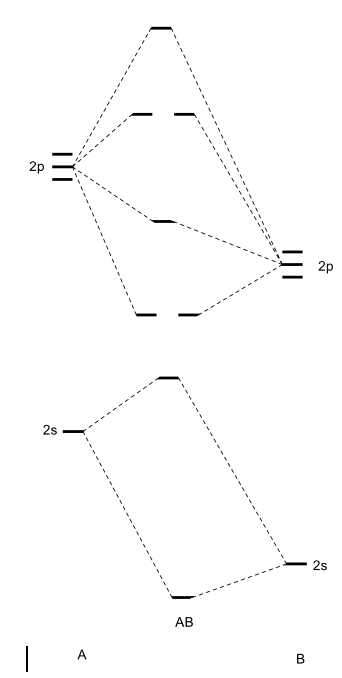


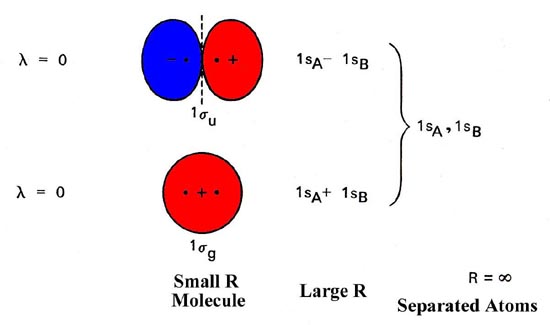



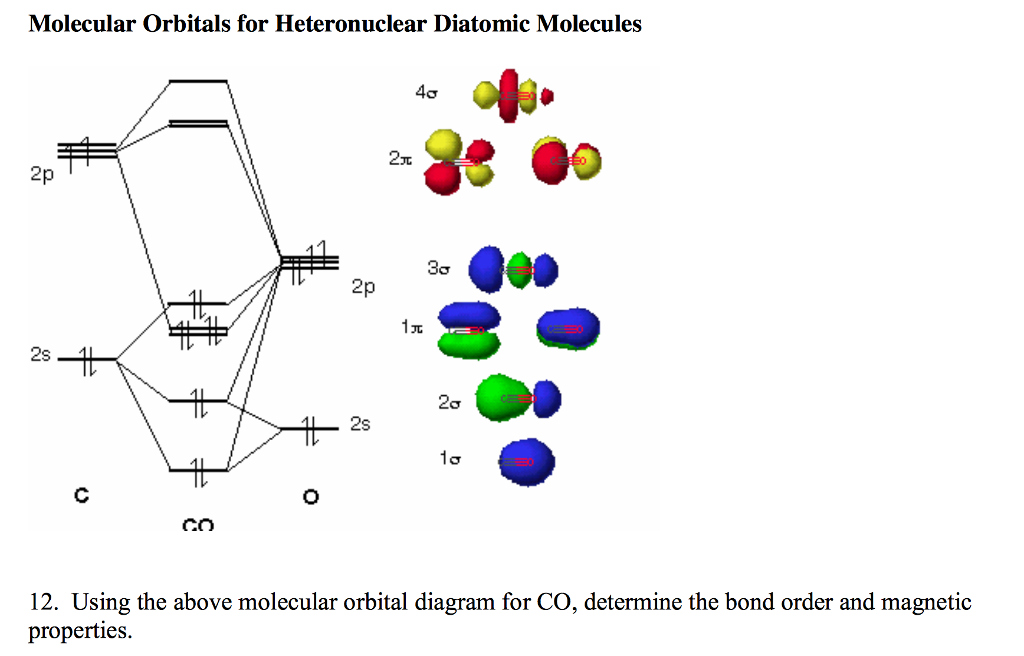
0 Response to "40 heteronuclear molecular orbital diagram"
Post a Comment