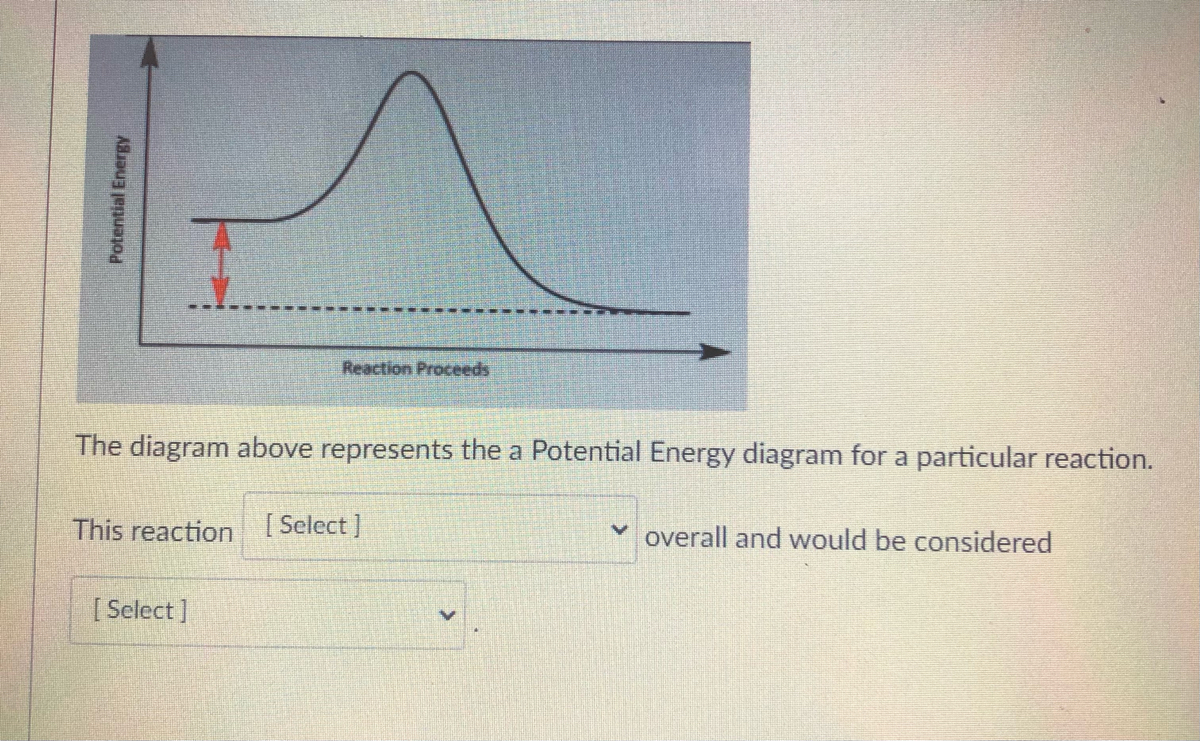
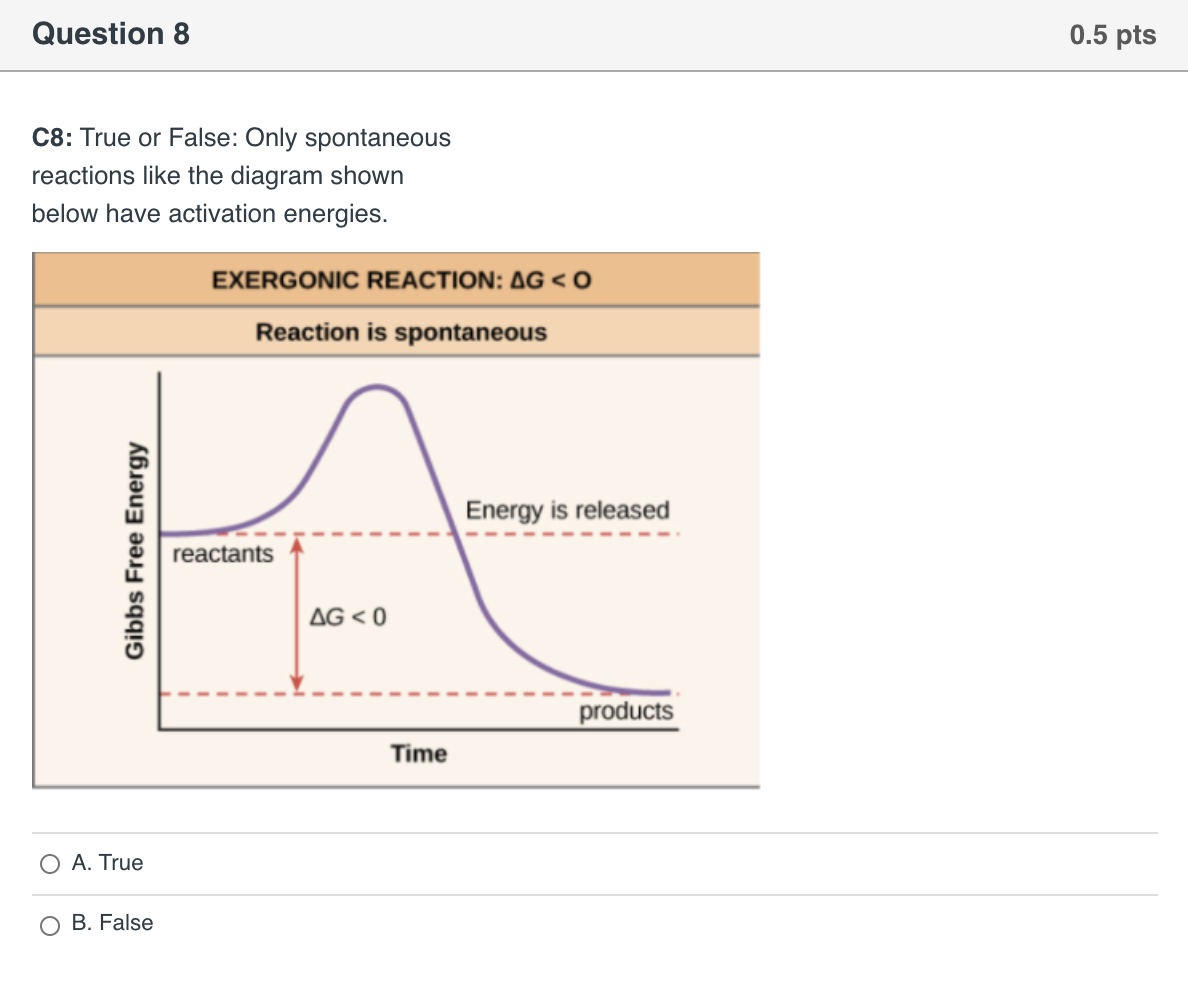
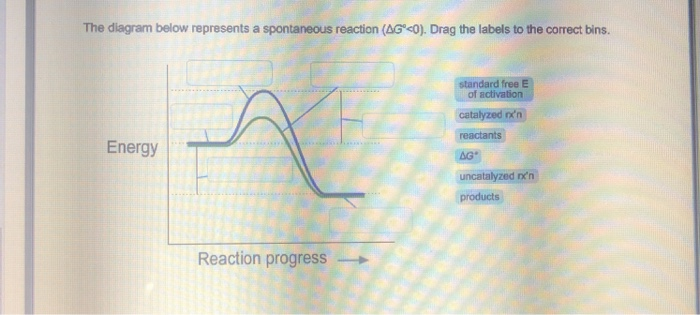
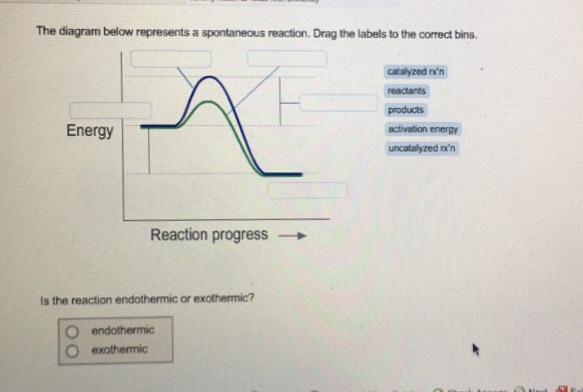
40 the diagram below represents a spontaneous reaction (δg
what does chup kar mean? Determine the enthalpy for this reaction: mgcl2(s)+2naoh(aq)→mg(oh)2(s)+2nacl(aq) Determine the value of h such that the matrix is the augmented matrix of a consistent linear system; Write an expression whose value is the character at index 3 of the str associated with s. The diagram below represents a spontaneous reaction (δg° country songs about brown eyes? Determine the enthalpy for this reaction: mgcl2(s)+2naoh(aq)→mg(oh)2(s)+2nacl(aq) Determine the value of h such that the matrix is the augmented matrix of a consistent linear system; Write an expression whose value is the character at index 3 of the str associated with s. The diagram below represents a spontaneous reaction (δg°
Thermodynamics Flashcards - Quizlet For the reaction below ΔG° = + 33.0 kJ, ΔH° = + 92.2 kJ, and ΔS° = + 198.7 J/K. Estimate ... The figure below represents the spontaneous reaction of H2 (shaded spheres) with O2 (unshaded spheres) to produce gaseous H2O. ... According to the diagram above, the forward reaction is. spontaneous at d, at equilibrium at e, and nonspontaneous ...

The diagram below represents a spontaneous reaction (δg
Gibbs free energy - Wikipedia The reaction will only be allowed if the total entropy change of the universe is zero or positive. This is reflected in a negative ΔG, and the reaction is called an exergonic process. If two chemical reactions are coupled, then an otherwise endergonic reaction (one with positive ΔG) can be made to happen. Solution: The diagram below represents a s... | Clutch Prep Watch the video solution for the question: The diagram below represents a spontaneous re... Subjects . Science ... Problem: The diagram below represents a spontaneous reaction (ΔG°<0). Fill in tthe blanks below FREE Expert Solution Show answer Answer: 98% (289 ratings) Solved The diagram below represents a spontaneous reaction ... The diagram below represents a spontaneous reaction (deltaG degree < 0). Drag the labels to the correct bins. uncatalyzed rx'n Delta G degree standard free E of activation products reactants catalyzed rx?n. Question: The diagram below represents a spontaneous reaction (deltaG degree < 0).
The diagram below represents a spontaneous reaction (δg. PDF CHM 152 Exam 4 Review Ch. 18 19 KEY j. Based on your answers from c, g and h, determine if the following reaction is spontaneous. Explain. 2Ag(s) + Cu2+(aq) → Cu(s) + 2Ag+(aq) No, the reaction a written above is not spontaneous. Based on the spontaneous reaction written in part c, Cu(s) is more likely to give up electrons and Ag+(aq) is more likely to accept electrons. ACS Omega | Vol 7, No 1 11-01-2022 · Two new pathways for controlling the size and shape of lead halide perovskite nanocrystals are presented. One is a modified hot injection method with centrifugation of a frozen eutectic mixture, and another is a low-temperature mixing and heat-up method. These new methodologies provide stable RGB primary color emission with pure halide perovskites. View … The Kb for methylamine, CH3NH2, at 25°C is ... - Clutch Prep By using the value of K b, calculate ΔG° for the equilibrium in part a. c. What is the value of ΔG at equilibrium? ... The reaction SO2(g) + 2H2S(g) ⇌ 3S(s) + 2H2O(g) is the basis of a suggested method for removal of SO2 from power-plant gases. ... The standard free... Q. The diagram below represents a spontaneous reaction (ΔG°<0 ... CHEM 4311 HW4 *in progress collab Flashcards - Quizlet The image represents a spontaneous, gaseous reaction at a constant temperature T KT K. Predict whether ΔHΔH, ΔSΔS, and ΔGΔG for this reaction are positive, negative, or zero. ... Consider these hypothetical chemical reactions: A⇌B,ΔG=A⇌B,ΔG= 10.5 kJ/molkJ/mol ... At 25 ∘C∘C the reaction from Part A has a composition as shown in ...
PDF 1. (18 points) making ethane thiolate: Derive the rate law ... The reaction given below is performed in water at a pH above the pKa of ethane thiol, thus ... the diagram. (3 pts) d) When ΔG° ... The kinetic order in water for the spontaneous hydrolysis reaction, n, and the hydronium ion catalyzed reaction, m, varies depending on the structure of the ... 30 The Following Diagram Represents The Reaction Of A (red ... The Following Diagram Represents The Reaction Of A Red Spheres With B2 ... 30 The Following Diagram Represents The Reaction Of A (red Spheres ... Solved: A. Write A Balanced Equation For The ReactionB. Would capitalize, underline, or italicize band names? Determine the enthalpy for this reaction: mgcl2(s)+2naoh(aq)→mg(oh)2(s)+2nacl(aq) Determine the value of h such that the matrix is the augmented matrix of a consistent linear system; Write an expression whose value is the character at index 3 of the str associated with s. The diagram below represents a spontaneous reaction (δg° The diagram below represents a spontaneous ... - HomeworkLib In energy profile diagram, if the reactants are at lower energy and products are at higher energy, then the reaction is non-spontaneous reaction.1 answer · 0 votes: Concepts and reason In energy profile diagram, if the reactants are at lower energy and products are at higher energy, then the reaction is non-spontaneous ...
Essentials of Physical Chemistry by B.S. Bahl ... - Academia.edu Preface The Essentials of Physical Chemistry has been written for BSc students. It has been national bestseller for more than 65 years. It has been used by more than 2 million students. It is 26 editions old. It really has been that long. A lot of PDF Chemical Equilibrium 7 - Glendale Unified School District A.The particle diagrams best represent that ΔH°<0 because the ions from both compounds are solvated by water molecules. B. The particle diagrams best represent that ΔH°<0 because both compounds produce about the same amount of CO 3 2− ions from the dissolution. C. The particle diagrams best represent that ΔS°>0 because both What is δg for this reaction?, the sign of the standard ... 3. ΔG°, or standard free energy, is the ΔG of a reaction at standard conditions, when each reactant is at a concentration of 1. The change in Gibb's energy of reaction, related to standard Gibb's energy is given by. ΔG = ΔG° + RT ln Q. At equilibrium ΔG = 0 and Q = K, thus equation becomes. Fluorescence Quenching - an overview | ScienceDirect Topics in which m is the number of CH 2 groups in the carbon chain and ΔG–CH 2 – ŝ the hydrophobic contribution made by each CH 2 group to the net driving force for the substrate into the electrical double-layer around the particle. A charged organic substrate can be gained to the surface by both electrostatic and hydrophobic means, a combined ...
Gibbs Free Energy | Boundless Chemistry - Lumen Learning Recall the condition for spontaneous change: ΔG = ΔH - TΔS < 0. where ΔG = change in Gibbs free energy, ΔH = change in enthalpy, T = absolute temperature, and ΔS = change in entropy. It is apparent that the temperature dependence of ΔG depends almost entirely on the entropy change associated with the process.
What is the opposite of "masculine"? Determine the enthalpy for this reaction: mgcl2(s)+2naoh(aq)→mg(oh)2(s)+2nacl(aq) Determine the value of h such that the matrix is the augmented matrix of a consistent linear system; Write an expression whose value is the character at index 3 of the str associated with s. The diagram below represents a spontaneous reaction (δg°
BIO 140 PASS_ W4S1.docx - Objectives: Students will be ... If a process is endergonic and non spontaneous, then what must be true? a. ΔS > 0 b. ΔH < 0 c. ΔG < 0 d. ΔS < 0 4. T/F: A spontaneous reaction energy is used. a. True b. False 5. The graph below represents which type of reaction?
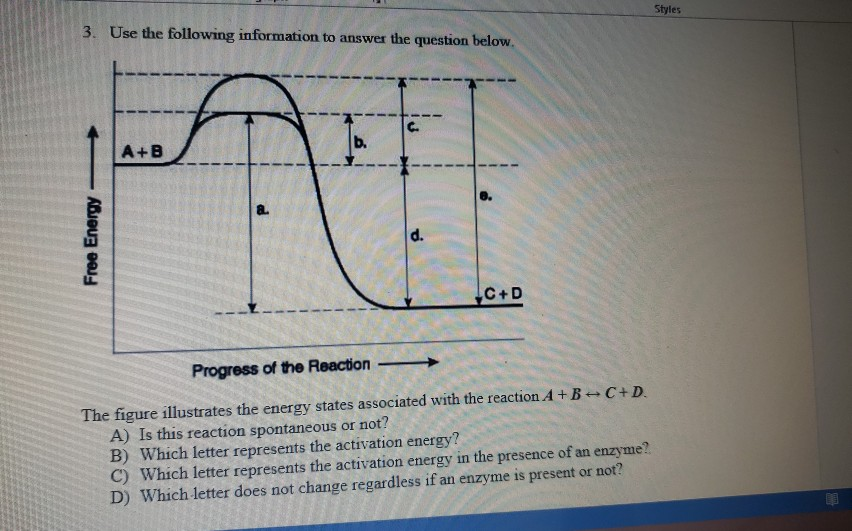
Solved The diagram represents a spontaneous reaction. Use ... 100% (24 ratings) Exothermic a …. View the full answer. Transcribed image text: The diagram represents a spontaneous reaction. Use the diagram to answer the questions below. Is the reaction endothermic or exothermic?
Gibbs free energy - chemguide ΔG = ΔH - TΔS. Remember that for a reaction to be feasible, ΔG has to be negative. ΔH could be negative (an exothermic reaction) or positive (an endothermic reaction). Similarly ΔS could be either positive or negative. There are four possible combinations of the signs of ΔH and ΔS. I want to look at those in turn.
CH103 – Chapter 7: Chemical Reactions in Biological Systems ... If the ΔG is negative the reaction is spontaneous, and if the ΔG is positive the reaction is not spontaneous. If ΔG = 0 the reaction is in a state of equilibrium, meaning that the forward reaction is happening at an equal rate as the reverse reaction and there is no net gain or loss of reactants and products.
Chapter 2: Protein Structure – Chemistry In the diagram below, ... The D- and L-Alanine enantiomer pair, upper diagram represents the ball and stick model and the lower diagram represents the line structure. Image (A) from NASA. ... Since it is known that protein folding is a spontaneous reaction, ...
Chem 180 Exam 3 Flashcards - Quizlet The image represents a spontaneous, gaseous reaction at a constant temperature T K. Predict whether ΔH, ΔS, and ΔG for this reaction are positive, negative, or zero. Acetylene, C2H2, can be converted to ethane, C2H6, by a process known as hydrogenation.
Solved The diagram below represents a spontaneous reaction Question: The diagram below represents a spontaneous reaction (deltaG < 0). Drag the labels to the correct bins. This problem has been solved! See the answer ...1 answer · Top answer: Left side must include REACTANTS right side (on the bottom) must be products, since they must be accordingly to the r...Missing: (δg | Must include: (δg
UG Question Papers & Answer Keys 17.01.2019 | PDF - Scribd Jan 17, 2019 · In a Conventional wing-aft tail airplane, the tail plane is located E (a) Below the horizontal level of the wing (b) Almost at the same horizontal level of the wing (c) Higher than the horizontal level of the wing (d) Arbitrarily as per design aerodynamics. 8. The relationship between the elastic constants E, G and K is given by
Answered: Consider the weak acid H2A and its… | bartleby Transcribed Image Text: Consider the weak acid H,A and its conjugate base HA-. Which diagram below represents a buffer solution? Explain your answer. = H2A = HA- A В. Expert Solution.
Gibbs Free Energy - Definition, Equations, 2nd Law of ... So change in Gibbs free energy is equal to the change in enthalpy minus the product of temperature and entropy change of the system. ΔG = ΔH - Δ (TS) If the reaction is carried out under constant temperature {ΔT=O} ΔG = ΔH - TΔS. This equation is called the Gibbs Helmholtz equation. ΔG > 0; the reaction is non-spontaneous and ...
KEYPRE-NATCHEM.docx - _1 Refer to the table below Which ... The potential energy diagram of the reaction is shown below. Which arrow represents the heat of reaction (ΔH) for the reverse reaction? ... When a reaction is exothermic and the products have more entropy than the reactants, the reaction is A. spontaneous, with a positive ΔG. Prepared by: Maria Michelle V. Junio and Esperanza R. Sabangan 1.
Solved The diagram below represents a spontaneous reaction ... The diagram below represents a spontaneous reaction (deltaG degree < 0). Drag the labels to the correct bins. uncatalyzed rx'n Delta G degree standard free E of activation products reactants catalyzed rx?n. Question: The diagram below represents a spontaneous reaction (deltaG degree < 0).
Solution: The diagram below represents a s... | Clutch Prep Watch the video solution for the question: The diagram below represents a spontaneous re... Subjects . Science ... Problem: The diagram below represents a spontaneous reaction (ΔG°<0). Fill in tthe blanks below FREE Expert Solution Show answer Answer: 98% (289 ratings)
Gibbs free energy - Wikipedia The reaction will only be allowed if the total entropy change of the universe is zero or positive. This is reflected in a negative ΔG, and the reaction is called an exergonic process. If two chemical reactions are coupled, then an otherwise endergonic reaction (one with positive ΔG) can be made to happen.



















0 Response to "40 the diagram below represents a spontaneous reaction (δg"
Post a Comment