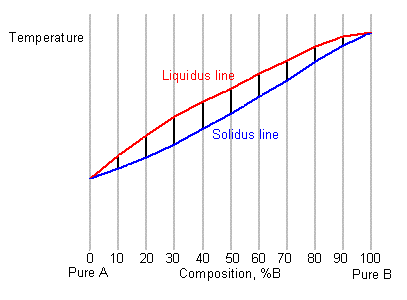
41 solidus line phase diagram
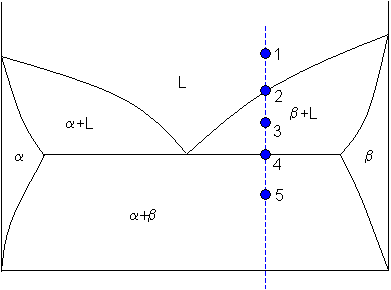
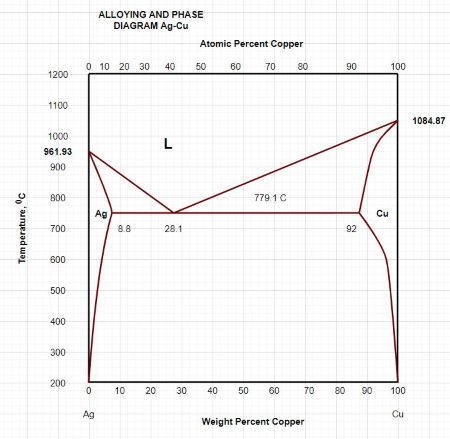
1. Locate composition and temperature in diagram . 2. In two phase region draw the tie line or isotherm . 3. Fraction of a phase is determined by taking the length of the tie line to the phase boundary for the other phase, and dividing by the total length of tie line . The lever rule is a mechanical analogy to the mass balance calculation. The tie line The solidus lines on a phase diagram represent the locus of all points that represent the completion of the solidification of a material of given composition as it is cooled in equilibrium from the liquid phase. For the copper-silver binary phase diagram shown …
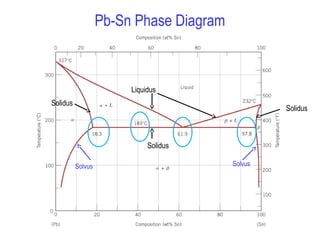
The solidus line (dotted line in Fig. 1) in the Sn-rich side of the Sn-In phase diagram remained indefinite until now. It was determined by measuring the chemical composition of solid-phase grains in samples annealed at different temperatures in the (liquid + γ) two-phase region.
Solidus line phase diagram
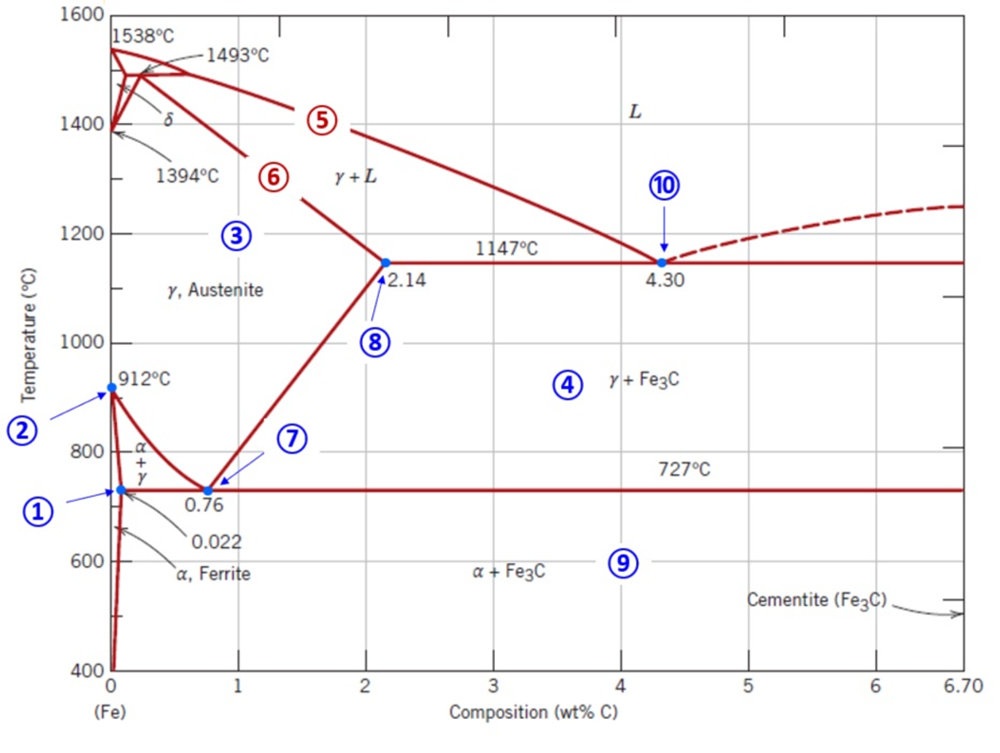
Solved 8. In the figure above you can see the phase diagram | Chegg.com. 8. In the figure above you can see the phase diagram for carbon and iron.a. Show where the liquidus line and the solidus line are in the phase diagram.b. Where is the eutectic point and what distinguishes it?c. Phase Diagrams: composition of phases At TA= 1320°C: Only Liquid (L) present CL= C0 ( = 35 wt% Ni) At TB= 1250°C: Both and L present At TD= 1190°C: Only Solid ( ) present C = C0( = 35 wt% Ni) C L = C liquidus ( = 32 wt% Ni) C = C solidus ( = 43 wt% Ni) 18 • Rule 3:If we know T and Co, then we know: --the amount of each phase (given in wt%). This phase diagram consists of two points, two lines and three areas. The two points of the two pure metals A & B. The upper line, obtained by connecting the points showing the beginning of solidification is called liquidius line, and the lower line, determined by connecting the points showing the end of solidification is called the solidus line.
Solidus line phase diagram. In Cigar shape diagram there are three different regions: 1. Liquid (single phase) 2. Liquid + solid (double phase) 3. Solid solution (single phase) •Liquidus- the boundary line between the liquid region and the double phase region. •Solidus- the boundary line between the solid solution region and the double phase region. Line in the phase diagram of an alloy system (Fig. 1) connecting all solidus temperatures and delimiting the temperature range in which the melt is only ... The phase diagram consists of two points, two lines, and three areas. The two points T A and T B represent the freezing points of the two pure metals. in 2-phase region: 1. Draw the tie line. 2. Note where the tie line intersects the liquidus and solidus lines (i.e. where the tie line crosses the phase boundaries). 3. Read off the composition at the boundaries: Liquid is composed of CL amount of Ni (31.5 wt% Ni). Solid is composed of Cαααα amount of Ni (42.5 wt% Ni).
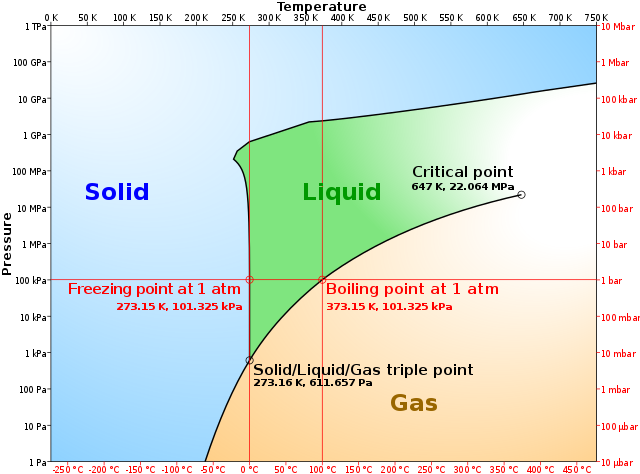
The tie line on a two-component solid- liquid phase diagram is an isothermal line that connects two equilibrium phases and passes through the alloy's position on the phase diagram when it is in a two-phase field, which intersects the two adjacent solubility curves. ... Note where the tie line intersects the liquidus and solidus lines (i.e ... A typical phase diagram. The solid green line applies to most substances; the dotted green line gives the anomalous behavior of water. The green lines mark the freezing point and the blue line the boiling point, showing how they vary with pressure. In chemistry, materials science, and physics, the solidus is the locus of temperatures (a curve on a phase diagram) below which a given substance is completely solid (crystallized). The solidus temperature, T S or T sol, specifies the temperature below which a material is completely solid, and the minimum temperature at which a melt can co-exist with crystals in thermodynamic equilibrium. The phase diagram for the Gd-Dy system as presented by Markova et al. showed six data points that formed a smooth curve defining the solidus of the system. This solidus has a slight downward curvature and the liquidus was drawn as a dashed straight line that connected the melting points of the end-members.
This is referred to as the solidus temperature, and the solidus line describes this temperature at varying compositions. The liquidous line describes the temperature at which the last little bit of solid melts at varying compositions. The eutectic line is a horizontal line drawn between two one-phase fields a the eutectic temperature. The phase diagram, therefore, consists of a liquidus line showing a minimum at the eutectic temperature, which is itself marked by a horizontal line. Since the solid phases formed consist simply of pure cadmium or pure bismuth, the solidus lines are coincident with the two vertical temperature axes. Sign in to download full-size image 1.14. The solidus line (dotted line in Fig. 1) in the Sn-rich side of the Sn-In phase diagram remained indefinite until now. It was determined by measuring the chemical composition of solid-phase grains in samples annealed at different temper-atures in the (liquid + c) two-phase region. The composition possible compositions of the alloy. The equilibrium "phase diagram" opposite shows the dependence of the alloys melting point on its composition, and the existence of the single solid phase below the solidus line indicates the complete solid state miscibility of the two elements. The phase diagram
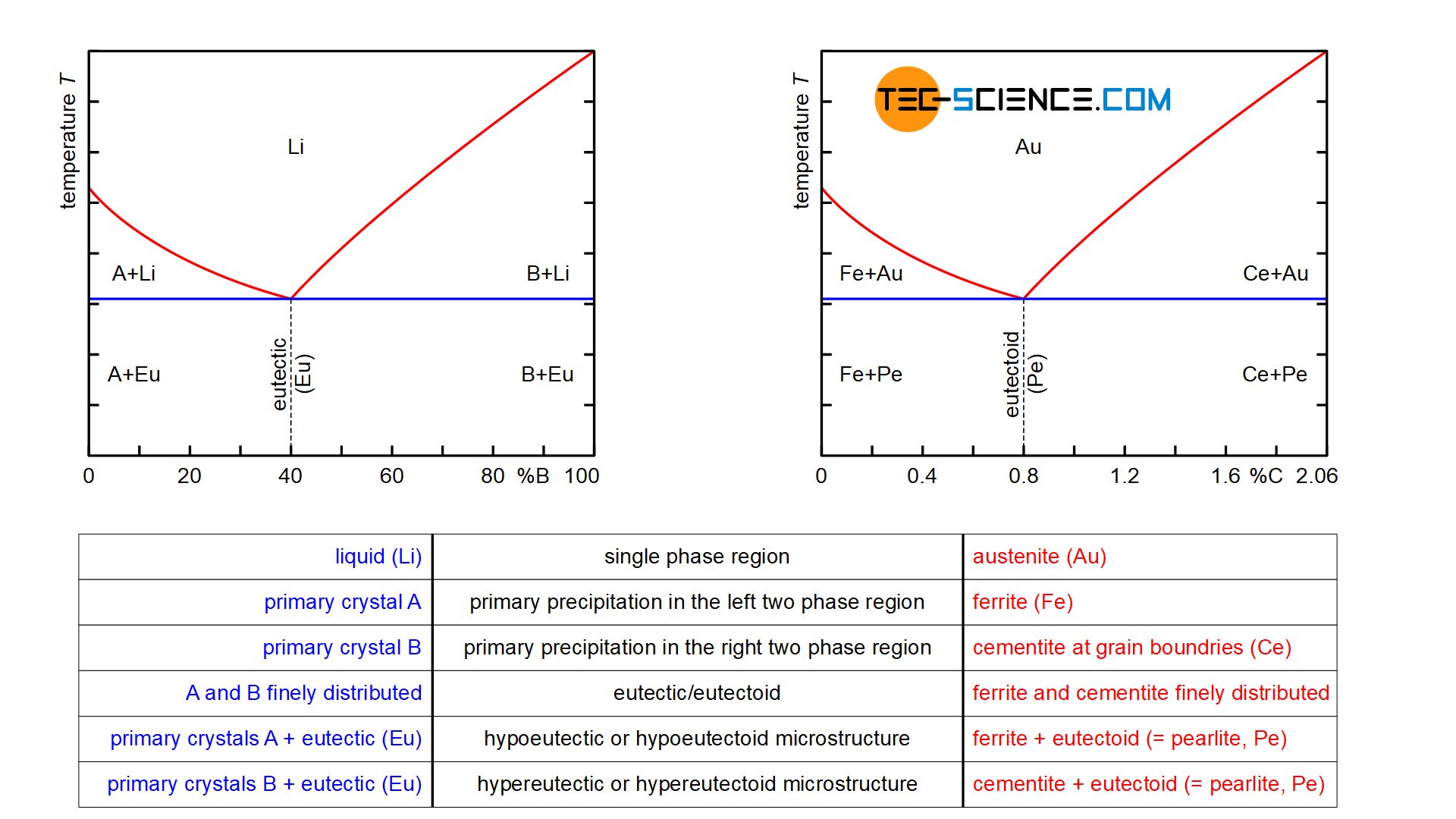
Between these two lines, a mixture of the metals is present in liquid and solid phases. A‘ A‘‘ E – primary crystal A (solid phase) and eutectic (liquid phase) B‘ B‘‘ E – primary crystal B (solid phase) and eutectic (liquid phase) When a eutectic alloy reaches the solidus line, i.e. the eutectic temperature, the solidification process is completed. The graph refers to a eutectic system composed of A and B where the shaded areas, namely the triangles A 'A ''E and B 'B'' E of ...
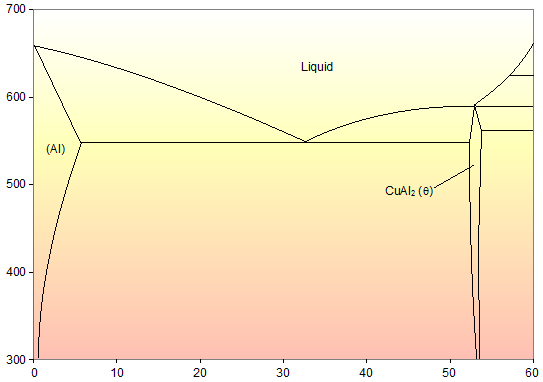
Solidus line is shifted to the right (higher Ni contents), solidification is complete at lower T, the outer part of the grains are richer in the low-melting component (Cu). • Upon heating grain boundaries will melt first. This can lead to premature mechanical failure. MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 22 Binary Eutectic Systems (I) systems …
Liquidus line of the Cu-Bi phase diagram and the metastable miscibility gap in the liquid state. Cu-Bi solidus and liquidus Data Figures - uploaded by Boris Straumal
The Solidus Line of the Cu-Bi Phase Diagram. Journal of Phase Equilibria, 1997. E. Rabkin. W. Gust. F. Sommer. E. Rabkin. W. Gust. F. Sommer. Download PDF. Download Full PDF Package. This paper. A short summary of this paper. 37 Full PDFs related to this paper. READ PAPER ...
As solidus line is a continuous line connecting the melting points of pure metals, the complete solidus line is MFGN. The phase diagram consists of four areas. The area above the liquidus line is a single-phase homogeneous liquid solution, since the two metals are soluble in the liquid state (labeled as Liquid solution).
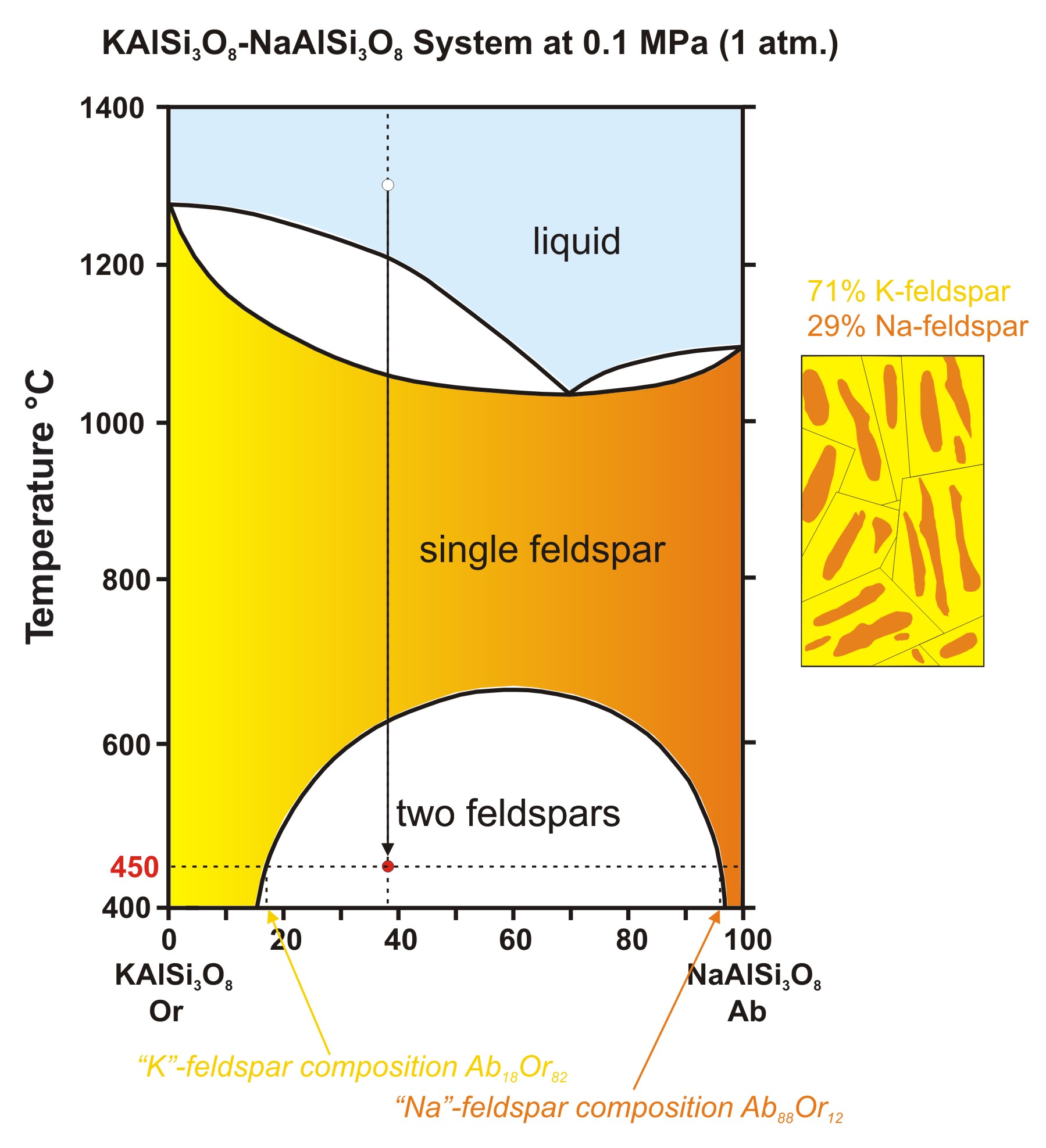
The solid solution phase diagram explains the behavior of chemical solid solution series, such as the transition from high temperature, calcium-rich plagioclase to low temperature sodium-rich plagioclase, or the transition from high temperature magnesium-rich to low temperature iron-rich crystals in ferromagnesium minerals (e.g. olivine, pyroxene).
Binary Metal System, Cadmium, Zinc, Tellurium, Phase Diagram, Solidus Line, Eutectic Transformation, Retrograde Solubility 1. Introduction The investigation of the equilibrium phase transformation in the twocomponent metal systems is among the - main problems of materials science.
The solid solubility of Bi in Cu single crystals has been experimentally determined. It is shown that the solidus line is a retrograde curve without a monotectic transition. The solid and liquid phases are successfully described with simple thermodynamic models. The experimentally measured maximum solubility of 0.0207 at. % Bi at 975 °C correlates well with that from the model (0.0193 at ...
The solvus is represented by a line on a phase diagram that separates a solid phase from a solid1 + solid2 phase, where solid1 and solid2 are different microstructures. The eutectic is represented by the horizontal line in a eutectic binary phase diagram, connecting the intersections of the solidus and solvus lines from both sides. The eutectic temperature also is where the liquidus lines for both components meet.
Journal of Phase Equilibria Vol. 18 No. 2 1997 The Solidus Line of the Cu–Bi Phase Diagram L.–S. Chang, B.B. Straumal*, E. Rabkin, W. Gust, and F. Sommer Max-Planck-Institut für Metallforschung Institut für Metallkunde der Universität Seestrasse 75, D–70174 Stuttgart, Germany (Submitted 18 September 1996; in revised form 8 November 1996)
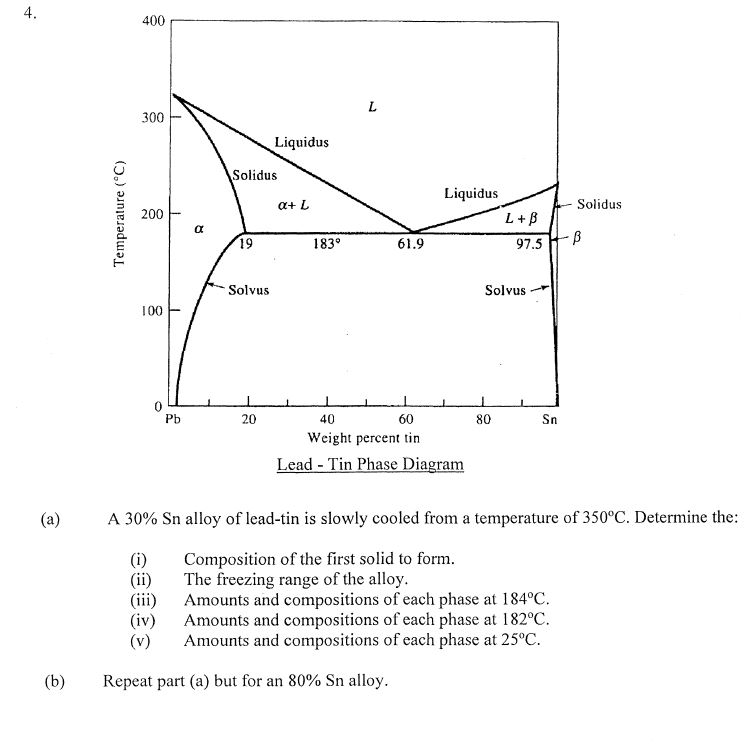
In this case the diagram is read the reverse of the crystallization steps (following the numbers below). 1. Imagine a rock containing 70 % B and 30% A. 2. It is slowly heated until it reaches the solidus line. At the solidus line the system shifts laterally to the eutectic point. 3.
3. The degree of freedom at triple point in unary diagram for water _____. (a) 0 (b) 1 (c) 2 (d) 3 4. Above the following line, liquid phase exist for all compositions in a phase diagram. (a) Tie-line (b) Solvus (c) Solidus (d) Liquidus 5. Following is wrong about a phase diagram. (a) It gives information on transformation rates.
Solidus- The line separating the field of all solid from that of liquid plus crystals. Eutectic point- the point on a phase diagram where the maximum number of allowable phases are in equilibrium. When this point is reached, the temperature must remain constant until one of the phases disappears. A eutectic is an
Binary Phase diagrams 9 Cooling of the alloy through two-phase zone Cooling of the 35%Ni alloy from the liquid to the solid α 1. As the 35%Ni liquid reaches the liquidus temperature the first solid forms with 46%Ni 2. At about 1250 ° C the liquid has 32%Ni and the solid 43%Ni 3. At the solidus line the last liquid has 24%Ni and the solid ...
line- The line on a phase diagram representing the limit of solid solubility as a function of temperature. Solidus line- The line on a phase diagram at which solidification is complete upon equilibrium cooling, or at which melting begins upon heating. Liquidus line-The line indicating above which melting of a solid is complete upon heating or ...
As nouns the difference between liquidus and solidus is that liquidus is (chemistry|physics) a line, in a phase diagram, above which a given substance is a stable liquid and below which solid and liquid are in equilibrium while solidus is...
John F. Smith, in Methods for Phase Diagram Determination, 2007. Publisher Summary. It was recognized that the terminal solubilities of impurities in a material differ between the liquid and solid states. In a phase diagram, this would be indicated by the separation between the liquidus and solidus lines. The fact that industry believes in the importance of phase diagrams is proven by the fact that industrial funding made the alloy phase diagram program possible.
This phase diagram consists of two points, two lines and three areas. The two points of the two pure metals A & B. The upper line, obtained by connecting the points showing the beginning of solidification is called liquidius line, and the lower line, determined by connecting the points showing the end of solidification is called the solidus line.
Phase Diagrams: composition of phases At TA= 1320°C: Only Liquid (L) present CL= C0 ( = 35 wt% Ni) At TB= 1250°C: Both and L present At TD= 1190°C: Only Solid ( ) present C = C0( = 35 wt% Ni) C L = C liquidus ( = 32 wt% Ni) C = C solidus ( = 43 wt% Ni) 18 • Rule 3:If we know T and Co, then we know: --the amount of each phase (given in wt%).
Solved 8. In the figure above you can see the phase diagram | Chegg.com. 8. In the figure above you can see the phase diagram for carbon and iron.a. Show where the liquidus line and the solidus line are in the phase diagram.b. Where is the eutectic point and what distinguishes it?c.


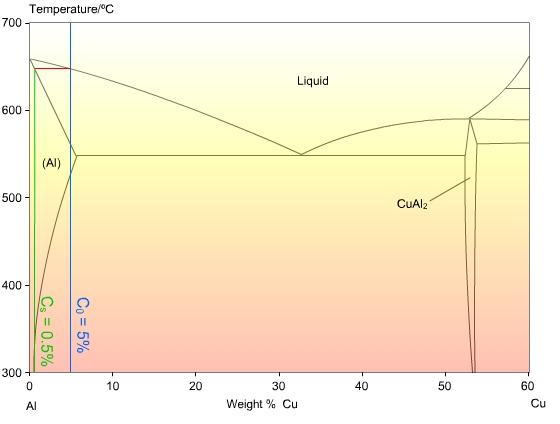


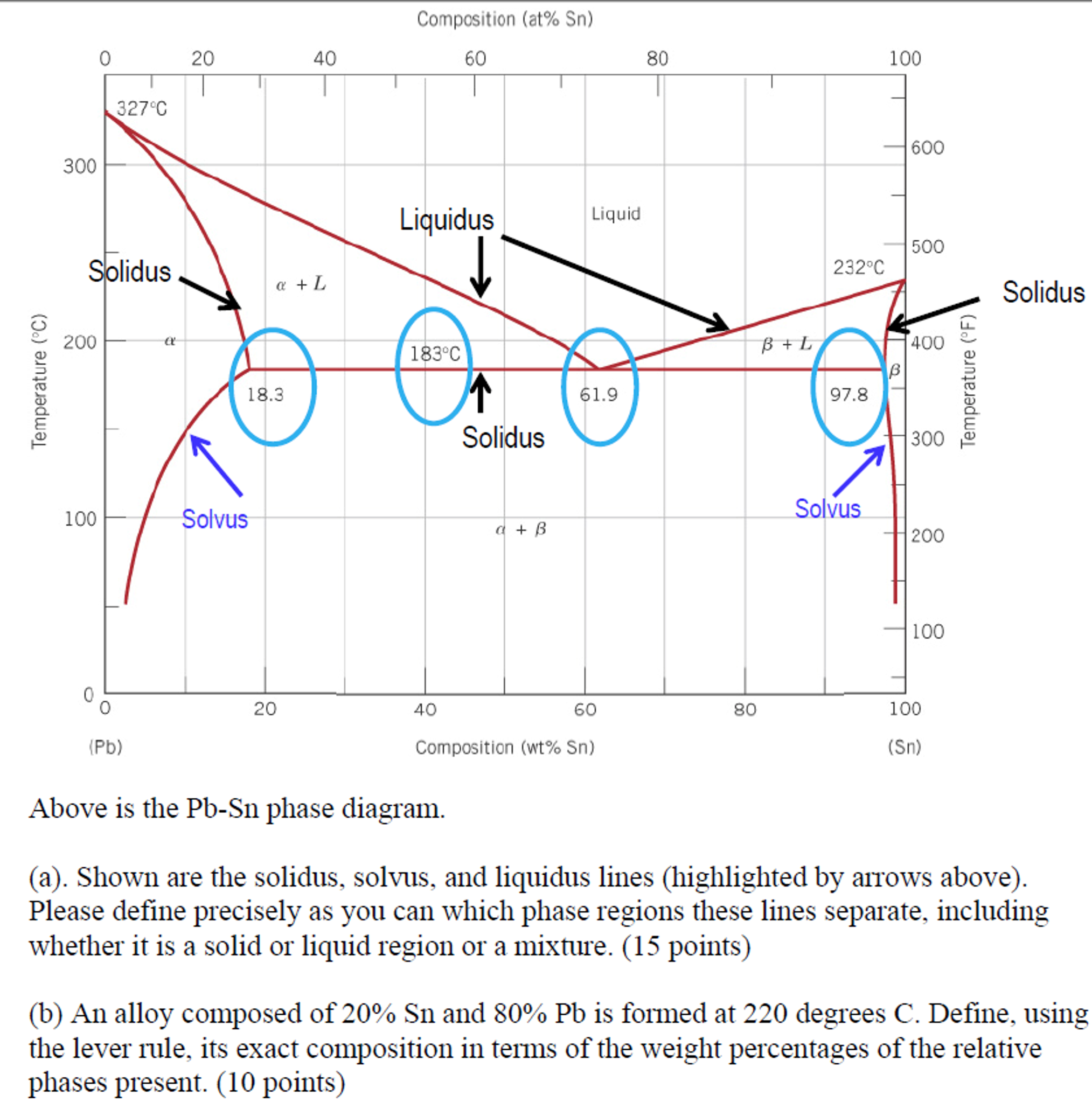


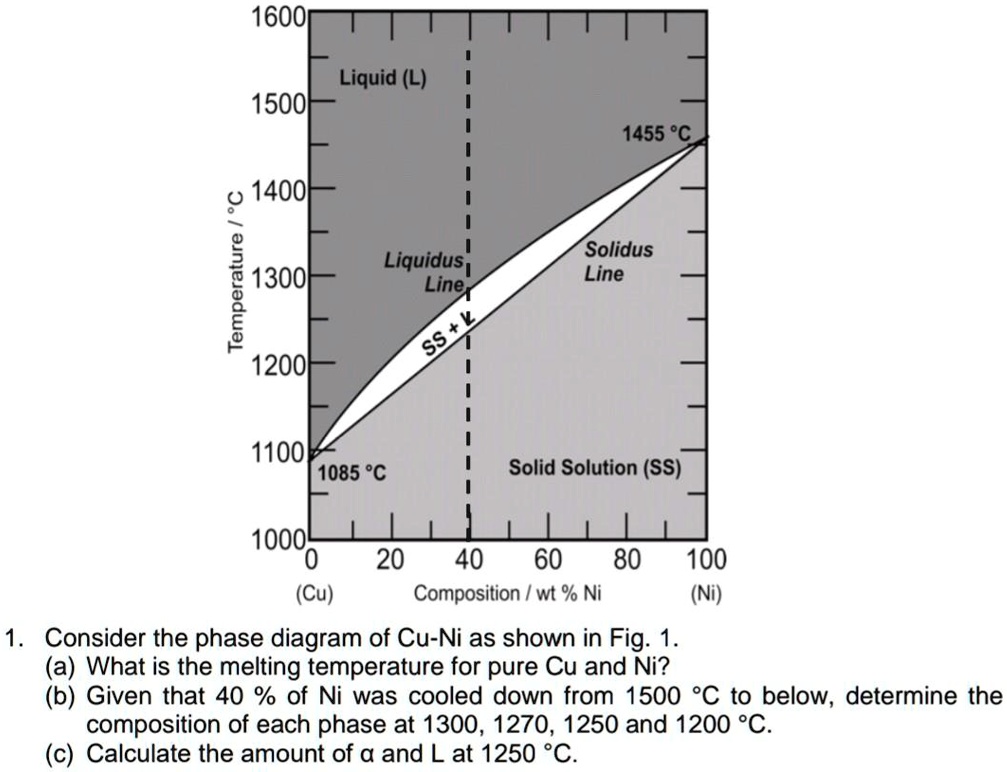

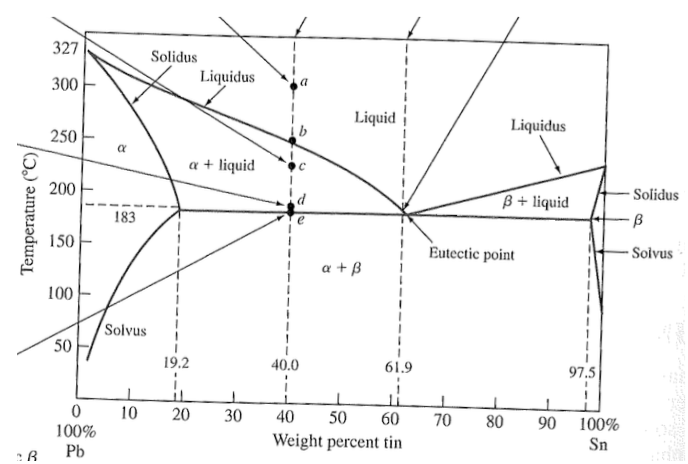












0 Response to "41 solidus line phase diagram"
Post a Comment