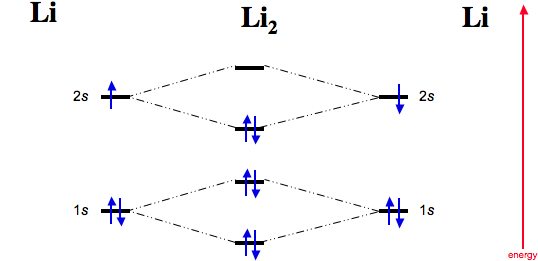
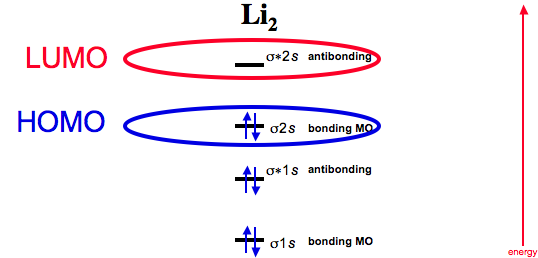
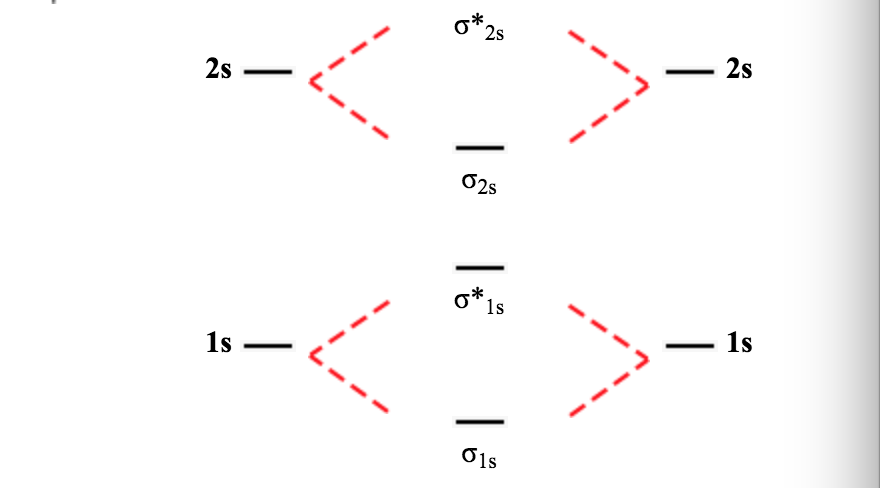
41 li2 molecular orbital diagram
For MO Diagram of Li22- see the below image The electron configuration of Li: 1s22s1 The electron configuration of Li View the full answer. Transcribed image text : Draw the molecular orbital (MO) electron Be sure your diagram contains all of the electrons in the ion, including any core electrons. Simple molecular orbital diagrams. Dihydrogen and its ion H2+. Dihelium He2. Dilithium Li2. As two H nuclei move toward each other, the 1s atomic orbitals of the isolated atoms gradually merge into a new molecular orbital in which the greatest electron density falls between the two nuclei.
...molecular orbital energy-level diagram for the H2 molecule. (b) The shapes of the molecular Factors that determine orbital interaction: ! energy difference between the interacting orbitals. 1. Homonuclear diatomic molecules such as Li2 utilize only F orbitals. For filled K shell bondin g and...

Li2 molecular orbital diagram
Nov 1, 2018 — Li2 Molecular orbital diagram. 2. See answers. Unlocked badge showing an astronaut's boot touching down on the moon.2 answers · 10 votes: I hope it's help you........dear A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. For example, homonuclear diatomic molecules of second row elements like Li2, Be2, B2 , C2, N2 , the σ 2pz MOs is higher in energy than π 2px and π 2py MOs. No. 9 Molecular Orbital Diagram for CO. Analysis done by Bond Order. If value of bond order is positive, it indicates a stable molecule and if...
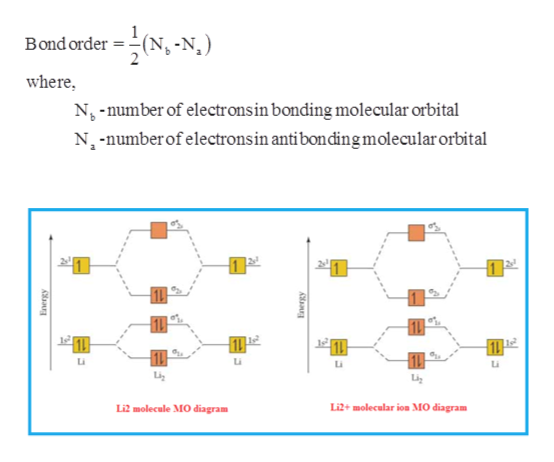
Li2 molecular orbital diagram. Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting Because both valence electrons would be in a bonding orbital, we would predict the Li2 molecule to Draw the molecular orbital diagram for the oxygen molecule, O2. From this diagram, calculate the... Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons. 3) If Nb = Na ,the molecule is again unstable because influence of electrons in the antibonding molecular orbital is greater than the bond influence of... A molecular orbital diagram shows both the energy of the atomic orbitals (from the atoms that are Figure 9.38 Molecular orbital diagram for species containing H and He Molecular Orbital Diagrams (Li2 - F2) The The molecular orbital diagram for the second row homonuclear. Chapter 9. Molecular Orbital Diagram (MO Diagram) of Li2. Смотреть позже. Поделиться.
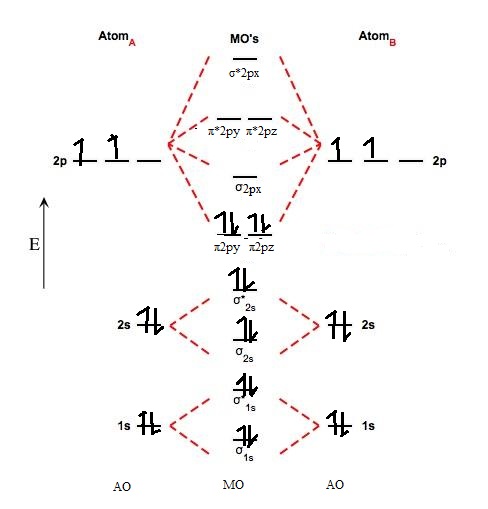
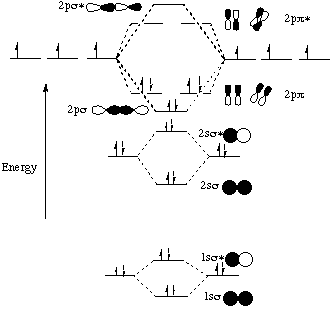
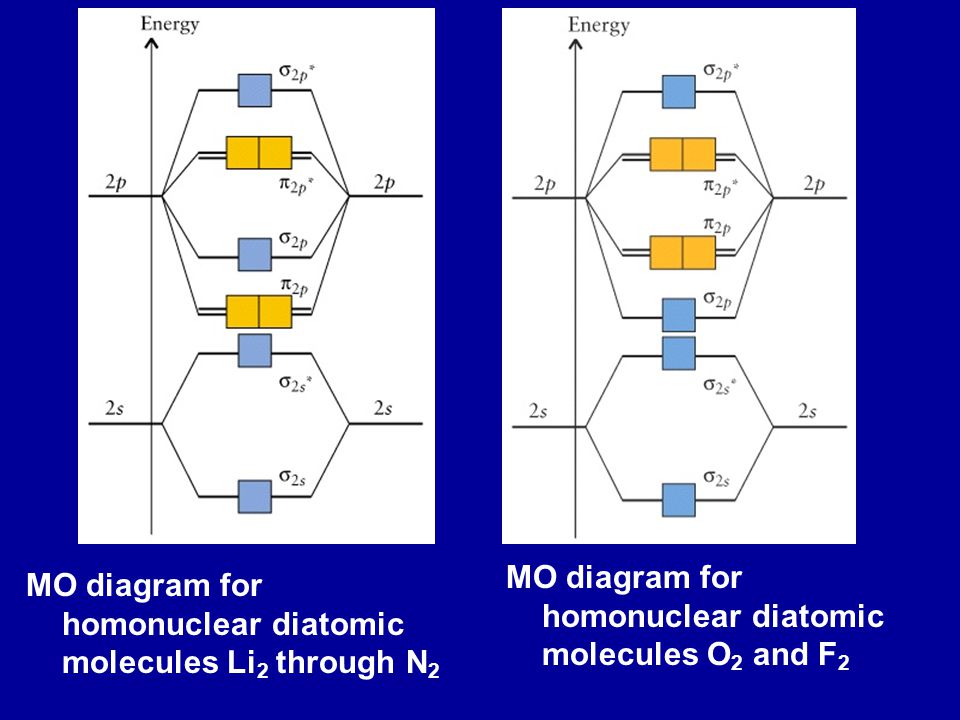
The procedure for working out a molecular orbital of a general diatomic molecule is quite simple. We construct molecular orbitals using the available orbitals on the atoms. We then fill up the molecular orbitals, starting with the lowest in energy, until all the electrons in the species have been assigned to... This video discusses how to draw the molecular orbital (MO) diagram for the Li2+ ion. The bond order of Li2+ is also calculated and the meaning of this... Let me explain the molecular orbital diagram of N2 using its diagram. one atom of nitrogen has 7 electrons so a N2 molecule will have 14 electrons. If we compare such diagrams for the diatomic molecules on the Second Period (Li₂, Be₂, B₂, C₂, N₂, O₂, and F₂), the resulting pattern looks like this Molecular orbital calculations indicate, however, that for O2, F2, and hypothetical Ne2 molecules, the 2p orbital is lower in energy than the 2p orbitals The solid lines represent the relative energies of the indicated atomic and molecular orbitals. (a) The diagram for H2, He2, Li2, Be2, B2, C2, and N2...
I have been taught that the MO diagram is different for molecules with 14 or less electrons than the one used for molecules with 15 or more electrons. Now note that even in this advanced molecular orbital theory a bunch of approximations is introduced, and the answer in general depends on at... A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent However, experimental and computational results for homonuclear diatomics from Li2 to N2 and certain heteronuclear combinations such as CO and... Generating molecular orbitals of molecules more complex than hydrogen using the LCAO method requires following a few additional guidelines Let's follow these guidelines and generate a molecular orbital electron configuration diagram for Li2 (Figure 9.21 "Molecular orbital electron configuration...
Valence bond (VB) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons.
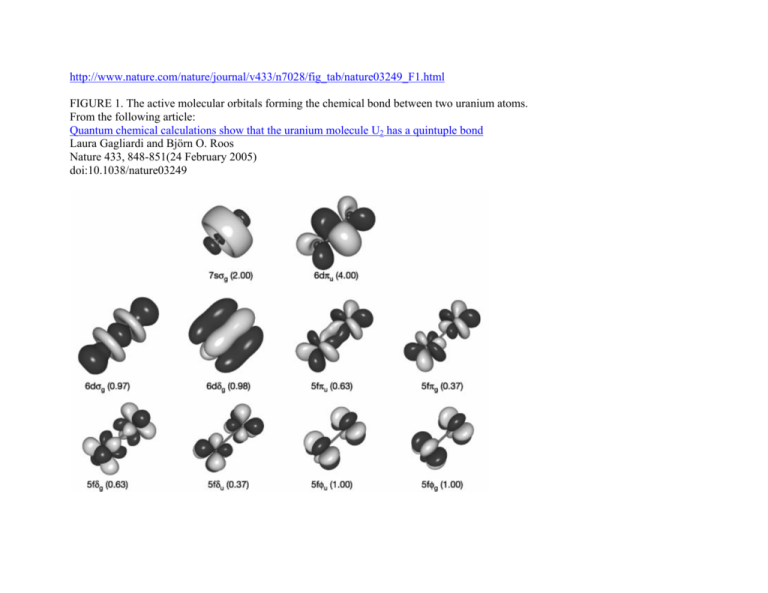
MOLECULAR ORBITAL and valence bond calculations of the w-electron energies of unsaturated molecules custom-arily start with models in which appropriate IN THE APPLICATION of molecular orbital theory to calculations of chemical binding energies, we shall use s e v e atom i a s follows: li
Use a qualitative molecular orbital energy-level diagram to predict the electron configuration, the Asked for: molecular orbital energy-level diagram, bond order, and number of unpaired electrons. We might therefore expect it to have similar reactivity as alkali metals such as Li and Na with their...
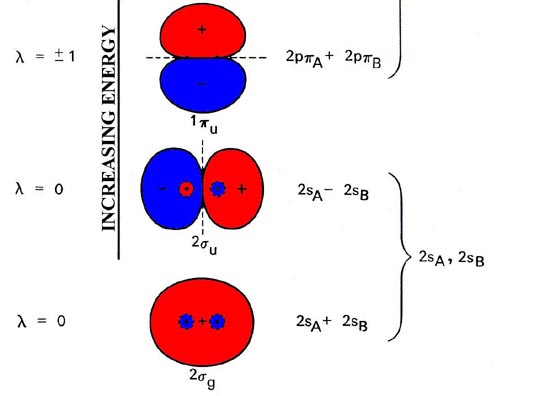
- Molecular orbital are formed by addition and subtraction of AO's. Æ Linear Combination of Atomic Orbitals (LCAO). - like hybrid AO's but the • Energy level diagram represents this interaction. - Two s orbitals interaction to create a low energy bonding and high energy anti-bonding molecular orbital.
We show an molecular orbital energy level diagram of LiH obtained by ab initio Hartree-Fock SCF-MO calculation with 6-311++G** basis set in this note. Since the 2s electron of Li comes close to the H atom, the 1s electrons of H in 2σorbital are destabilized by electron repulsion.
Molecular Orbital energy level Diagrams of Li2 according to the molecular orbital theory explains the bond order and magnetic ... This video discusses how to draw the molecular orbital (MO) diagram for the Li2 ion. The bond order of Li2 is also calculated ...
Molecular orbital : A molecule in which all the electrons are paired, is called diamagnetic. | Online Chemistry tutorial IIT, CBSE Chemistry, ICSE Chemistry, engineering and medical chemistry entrance exams Molecular orbital diagram of C2 molecule : Number of electrons in C2 molecule = 12.
The molecular orbital energy diagram predicts that He2 will not be a stable molecule, since it has equal numbers of bonding and antibonding Eight possible homonuclear diatomic molecules might be formed by the atoms of the second period of the periodic table: Li2, Be2, B2, C2, N2, O2, F2, and Ne2.
Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. If you can understand the foundation and skeleton of the diagram specific to that molecule, then it will be easier and faster for you to draw 2nd period. Li-Be. B,C,N- note difference in order of p orbitals from O,F,Ne.
The energy levels of these molecular orbitals have been determined experimentally by various methods. The order of increasing energy of For molecules Li2, Be2, B2, C2 and N2 the molecular orbital energy level diagram. In the diagram, the molecular orbitals are place at the center and the...
Draw a molecular orbital diagram for He2, calculate the bond order and determine its stability. Show the shapes of the molecular orbitals also. -There is a big gap in energy between 1s and 2s orbitals in lithium -On the diagram, horizontal lines indicate atomic orbitals -Numbers on the lines indicate the...
For example, homonuclear diatomic molecules of second row elements like Li2, Be2, B2 , C2, N2 , the σ 2pz MOs is higher in energy than π 2px and π 2py MOs. No. 9 Molecular Orbital Diagram for CO. Analysis done by Bond Order. If value of bond order is positive, it indicates a stable molecule and if...
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
Nov 1, 2018 — Li2 Molecular orbital diagram. 2. See answers. Unlocked badge showing an astronaut's boot touching down on the moon.2 answers · 10 votes: I hope it's help you........dear





















0 Response to "41 li2 molecular orbital diagram"
Post a Comment