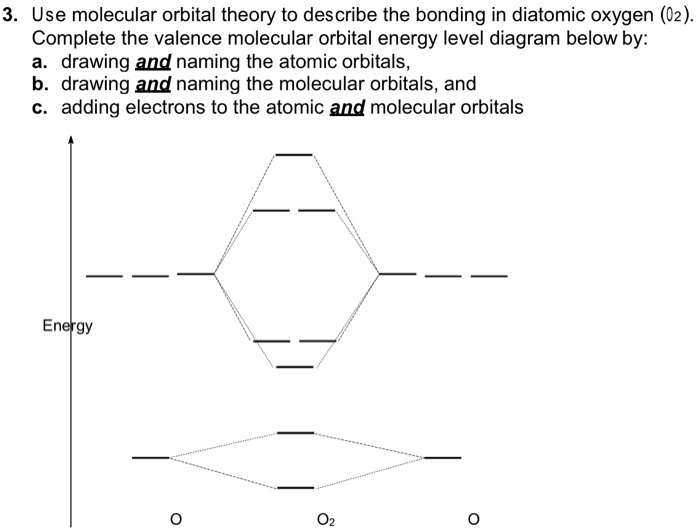
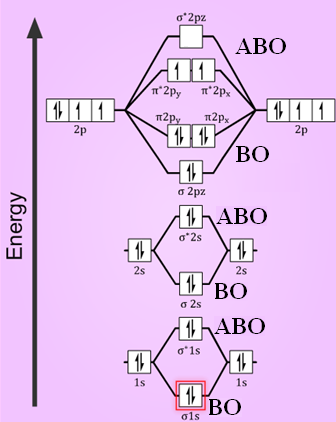
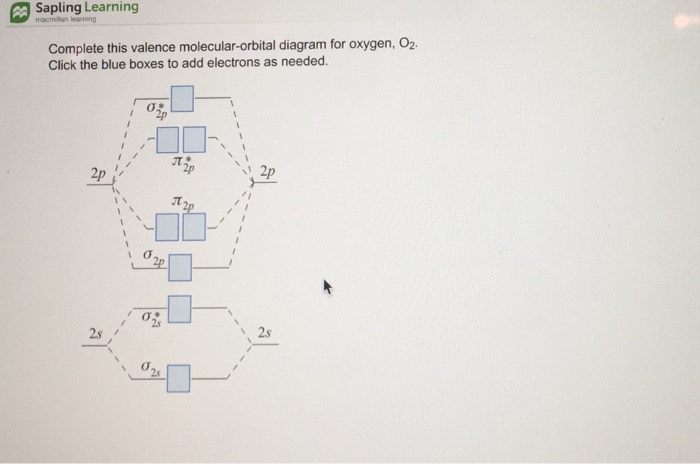
39 complete this valence molecular-orbital diagram for oxygen o2
(PDF) Inorganic Chemistry Housecroft | Yurika Almanda ... Pembuatan Senyawa kompleks asetial asetanoat. Enter the email address you signed up with and we'll email you a reset link. (Get Answer) - Sketch the Molecular Orbital diagram for O2 ... Sketch the Molecular Orbital diagram for O2 being sure to: A. Designate bonding and anti-bonding orbitals, B. Show the location of all electrons in the molecular orbitals, C. Calculate the bond order, and D. Indicate whether this molecule is paramagnetic or diamagnetic. For your diagram, assume that one oxygen atom contributes its six (6) valence electrons to the molecule and the other oxygen contributes seven (7) valence electrons to the molecule.
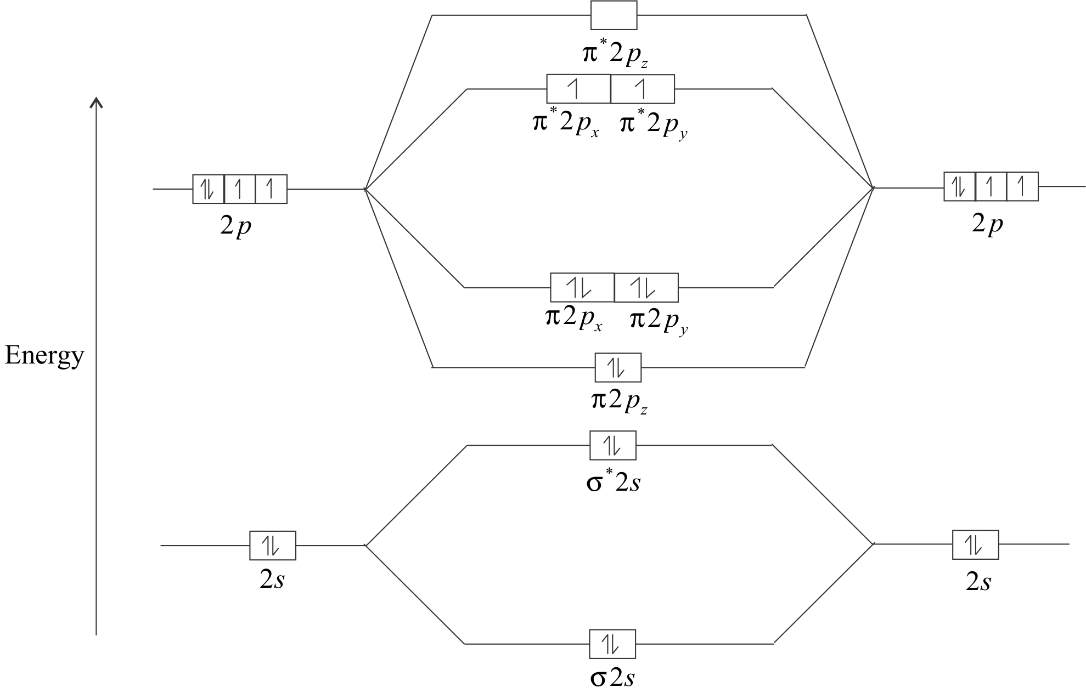
Question #84799 + Example - Socratic.org They are shown in red in the above diagram. 2 in the σ* 1s MO 2 in the σ* 2s MO 1 in the π* 2py MO 1 in the π* 2pz MO This gives you a total of 6 anti-bonding electrons. The bond order for the oxygen molecule will thus be B.O. = 1 2 ⋅ (10 − 6) B.O. = 1 2 ⋅ 4 = 2

Complete this valence molecular-orbital diagram for oxygen o2
PDF Miessler-Fischer-Tarr5e SM Ch 05 CM number of valence electrons) with O2. Therefore, NF is predicted to be paramagnetic with a bond order of 2. The populations of the bonding (8 electrons) and antibonding (4 electrons) molecular orbitals in the diagram suggest a double bond. c. The 2s, 2s *, 2p, and 2p * orbitals exhibit C v symmetry, with the NF bond axis the infinite-fold Explain the formation of O2 molecule using molecular ... The molecular orbital energy level diagram of oxygen molecule is given as follows : Bond order 2 N b − N a = 2 8 − 4 = 2 Thus, oxygen molecule has two bonds. i.e., one is bond and one p bond. smartbook 14 Flashcards - Quizlet molecular orbitals can be formed by the combination of multiple atomic orbitals, allowing electrons to be _____ or shared between several atoms. the molecular orbital model therefore allows a better description of the bonding in _____ structure than valence bond theory, which depicts electrons as being _____ between two atoms at a time
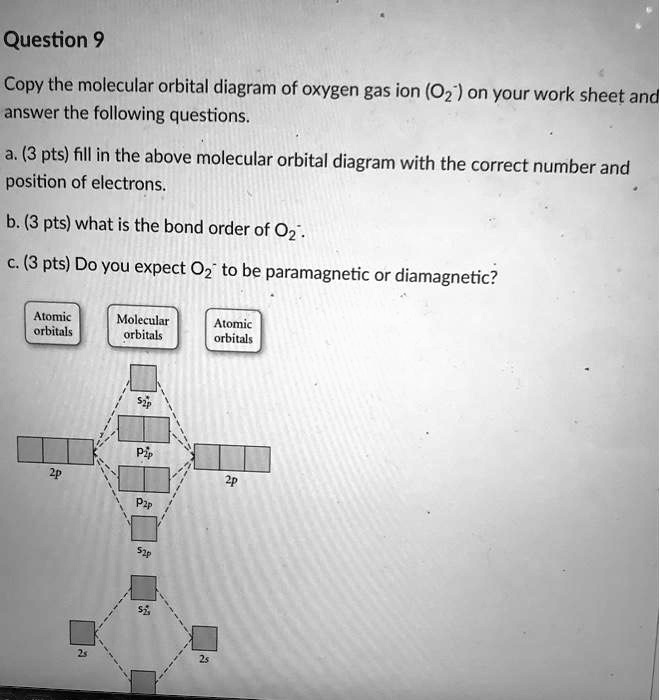
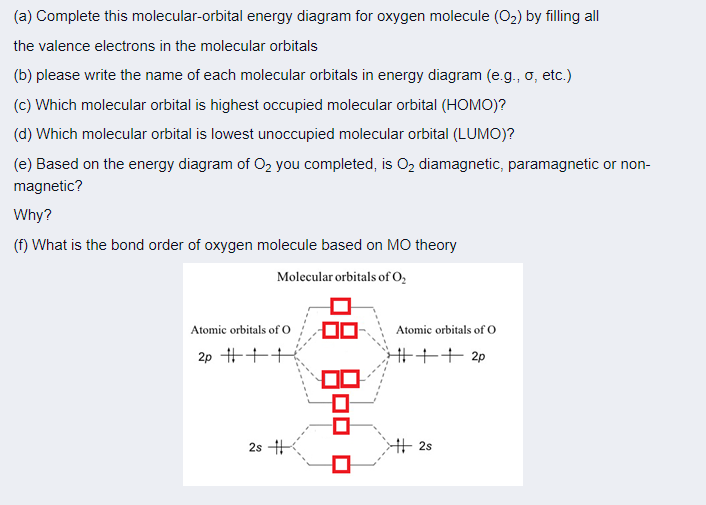
Complete this valence molecular-orbital diagram for oxygen o2. Explain the formation of O2 molecule using molecular class ... The molecular orbital diagram of an Oxygen molecule is as - From the diagram, the electronic configuration of oxygen molecule can be written as - ${O_2}$ :- $\sigma 1{s^2}{\sigma ^*}1{s^2}\sigma 2{s^2}{\sigma ^*}2{s^2}\sigma 2{p^2}\Pi 2p_x^2\Pi 2p_y^2{\Pi ^*}2p_x^1{\Pi ^*}2p_y^1$ Solved (a) Complete this molecular-orbital energy diagram ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: (a) Complete this molecular-orbital energy diagram for oxygen molecule (O2) by filling all the valence electrons in the molecular orbitals (b) please write the name of each molecular orbitals in energy diagram (e.g., o, etc.) (c) Which molecular orbital is highest occupied molecular orbital (HOMO)? Molecular Orbital Theory - Purdue University The only orbitals that are important in our discussion of molecular orbitals are those formed when valence-shell orbitals are combined. The molecular orbital diagram for an O 2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions between the 2s and 2p valence orbitals. a) Construct an MO diagram for the formation of O2; use ... Sketch the Molecular Orbital diagram for O2 being sure to: A. Designate bonding and anti-bonding orbitals, B. Show the location of all electrons in the molecular orbitals, C. Calculate the bond order, and D. Indicate whether this molecule is...
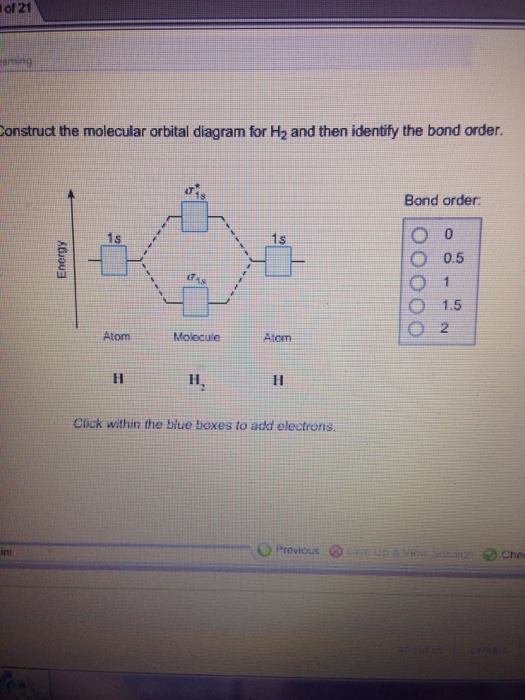
molecular orbital diagram for o2 - penguinskiclub.org Penguin Ski Club of New Hampshire. Located in Lincoln NH near Loon Mountain. Menu and widgets Ab Initio Simulations of Water/Metal Interfaces | Chemical ... Structures and processes at water/metal interfaces play an important technological role in electrochemical energy conversion and storage, photoconversion, sensors, and corrosion, just to name a few. However, they are also of fundamental significance as a model system for the study of solid–liquid interfaces, which requires combining concepts from the chemistry and physics of … Coursework Hero - We provide solutions to students Our writers can complete a standard essay for you within 1-3 hours and a part of a dissertation – in 2-5 days. High Quality. All the papers we deliver to clients are based on credible sources and are quality-approved by our editors. Next. Why Customers Become Our Regulars We put decades of writing experience to work for you and are passionate about helping you succeed. Let the … Construct the molecular orbital diagram fo... | Clutch Prep Construct the molecular orbital diagram for He 2 and then identify the bond order. Click within the blue boxes to add electrons. Bond order: a) 0. b) 0.5. c) 1. d) 1.5. e) 2. Learn this topic by watching MO Theory: Bond Order Concept Videos.
42 complete this molecular orbital diagram for cn - Wiring ... Question: Complete the molecular orbital diagram for CN. Note that the 1s orbitals are not shown. Identify the bond order of CN. O2 01 OOOOO 25- 0 2s Answer Bank The atomic orbitals on the left side of the molecular orbital diagram are those of The atomic orbitals on the right side of the molecular orbital diagram are those of. Complete this valence molecular-orbital diagram for oxygen ... FREE Answer to Complete this valence molecular-orbital diagram for oxygen, O2. Click the blue boxes to add electrons as...1 answer · Top answer: Concepts and reason The concept used to solve this problem is based on molecular orbital diagram. A molecular orbital diagram is used to explain ... Complete this valence molecular-orbital diagram for oxygen ... 13. (14 pts) MO Theory Draw the complete (core and valence) molecular orbital energy level diagram for the homonuclear diatomic molecule Be2. Use standard MO symbols to label the energy levels (That is: o, o, , or n*, as needed, with subscripts indicating which atomic orbitals formed them.) a. oxygen fluoride molecular orbital diagram oxygen fluoride molecular orbital diagram. Posted on February 20, 2022 by ...
NCERT Xtract - Objective Chemistry.pdf - Chemistry Class ... Liquid benzene (C6H6) burns in oxygen according to the, equation 2C 6 H 6 (l ) 15O 2 ( g ), 12 CO 2 ( g ) 6 H 2 O ( g ), How many litres of O2 at STP are needed to complete the, combustion of 39 g of liquid benzene?(Mol. wt. of O2 = 32,, C6H6 = 78), (a) 74 L, (b) 11.2 L, (c) 22.4 L, (d) 84 L, 143. Assuming fully decomposed, the volume of CO2 released at, STP on heating 9.85 g of …
40 f2- molecular orbital diagram The orbital diagram for a diatomic molecule is. To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels in the diagram) one at a time until you have used them up. They completely fill all the orbitals except the highest-energy antibonding sigma 2p orbital.
Molecular Orbital (MO) Diagram of O2 - YouTube Molecular Orbital Diagram for Oxygen Gas (O2).Fill from the bottom up, with 12 electrons total.Bonding Order is 2, and it is Paramagnetic.sigma2s(2),sigma2s*...
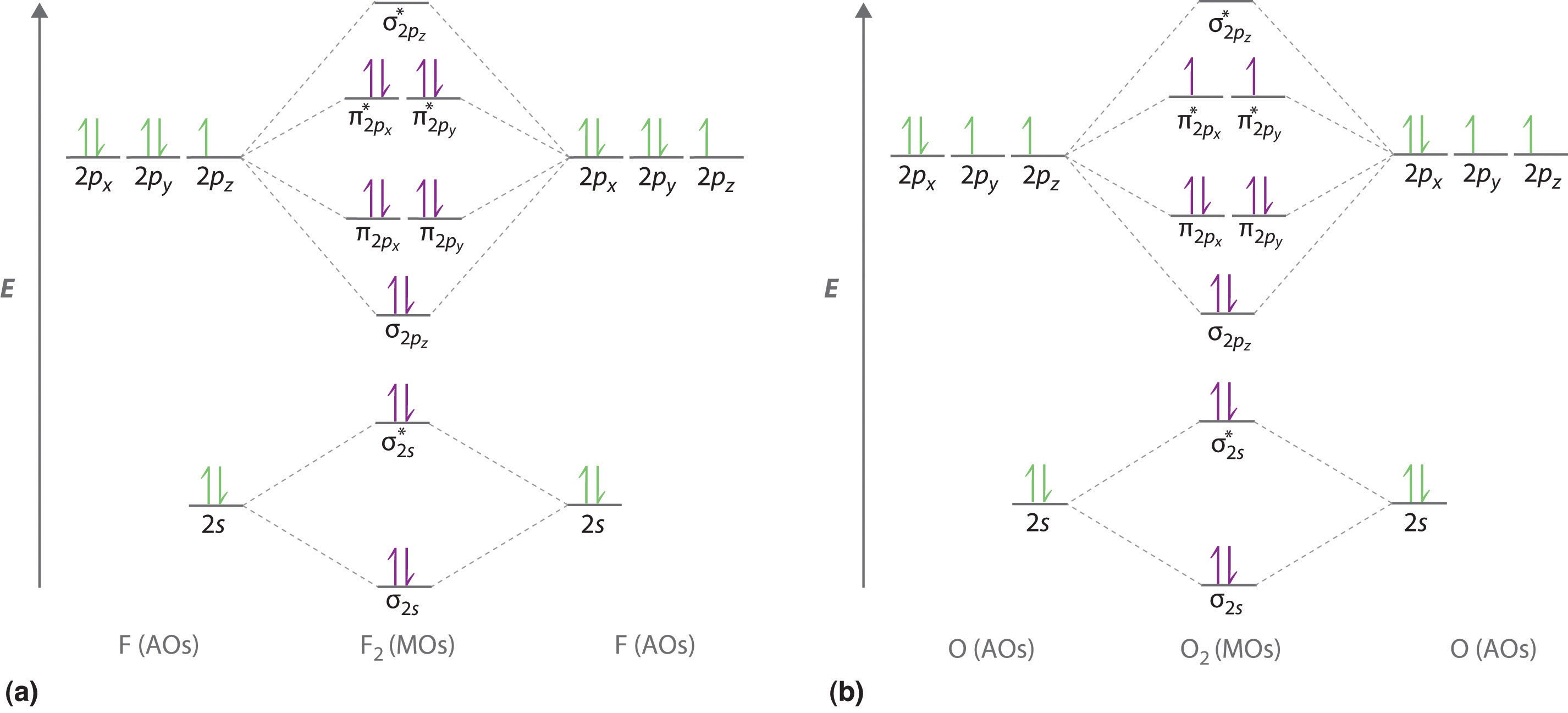
PDF MO Diagrams for Diatomic Molecules molecular electron configuration for O2 σ2σ*2σ2π4π*2 We can also calculate the O-O bond order: BO 1 2 # bonding e # anti-bonding e 1 2 8 4 2 LCAO MO theory also predicts (correctly) that O2has two unpaired electrons.
Special Case of Highly Electronegative Elements Atomic oxygen has 6 valence electrons and 4 valence orbitals (2s, 2px, 2py, and 2pz). We can draw a Lewis structure of molecular oxygen with a double bond between the oxygen atoms and 2 non-bonding pairs of electrons on each atom. However, experimentally we can determine that O2has 2 unpaired electrons.
O2 Lewis Structure, Molecular Geometry, and Hybridization As we studied above that one oxygen atom has a deficiency of two valence electrons, it readily accepts two electrons. So, a single oxygen molecule has six electrons in its octet. If we look for O2, then the number will be O2: 6+6 = 12. In total, an O2 molecule needs four valence electrons to complete its octet and achieve a stable condition.
Is O2 ionic or covalent? - Techiescientist O2 is a covalent molecule because each oxygen atom needs two valence electrons to complete its octet. To meet this need, each oxygen atom shares two of its electrons with the other oxygen forming a strong oxygen-oxygen double shared covalent bond.
solucionario quimica de raymond chang 12 ... - Academia.edu solucionario quimica de raymond chang 12 edicion . 697 Pages. solucionario quimica de raymond chang 12 edicion
How do yo write the orbital diagram for oxygen? | Socratic The electron configuration for oxygen is: 1s^2 2s^2 2p^4 This video will walk you through the step of writing orbital diagram. The video uses Kr as an example, but the process is exactly as the same as what you need to do for oxygen. Hope this helps!
Solved Complete this valence molecular-orbital diagram for ... Complete this valence molecular-orbital diagram for oxygen, O2. Click the blue boxes to add electrons as needed. Question: Complete this valence molecular-orbital diagram for oxygen, O2. Click the blue boxes to add electrons as needed.
Chapter 14 Quiz Flashcards | Quizlet The fact that O2 is paramagnetic can be explained by: a) hybridization of atomic orbitals in O2. b) the Lewis structure of O2. c) the molecular-orbital diagram for O2. d) resonance. e) a violation of the octet rule.
P orbital diagram | riesige auswahl an cds, vinyl und mp3s As two atoms approach each other, their electron orbitals begin to overlap. This overlap forms a molecular bond between the two atoms with its own molecular orbital shape Question: Complete This Valence Molecular-orbital Diagram For Oxygen, O2. Click The Blue Boxes To Add Electrons As Needed. This problem has been solved! See the answer.
What is the orbital diagram of oxygen? 4.1/5 (2,285 Views . 10 Votes) In writing the electron configuration for oxygen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for O go in the 2s orbital. The remaining four electrons will go in the 2p orbital. Therefore the O electron configuration will be 1s22s22p4.
Why is O2 paramagnetic class 11 chemistry CBSE The valence bond theory could not explain the paramagnetic nature of oxygen molecules. This is where the molecular orbital comes into picture. Based on valence bond theory, the electronic configuration of an atom of oxygen is $1{s^2}2{s^2}2{p^4}$. Valence bond theory explains that the bonds are formed by atomic orbital and because of this, electrons are paired at the course of overlapping and so, oxygen is a diamagnetic species.
(PDF) Accurate ab initio potential energy curve of O2. II ... The embedding CI space is that of 12 electrons in the full space of all 8 valence orbitals FORS12/8. Prediction of the six highest vibrational levels for the 3 g − ground electronic state of O 2 ...
MO Orbital Lab - Part I: MO diagram of oxygen molecule, O2 ... View full document. Part I: MO diagram of oxygen molecule, O2 Below is the energy level diagram found from GaussView of an O2 molecule: Figure 1: O2 Molecule Energy Level Diagram One neutral oxygen atom has a total of 8 electrons, and an O2 molecule contains 16 electrons. The 1s orbital holds a total of 4 electrons, which 2 electrons go into the bonding orbital and 2 electrons go into the antibonding orbital.
Oxygen(O) electron configuration and orbital diagram Orbital Diagram for Oxygen (O) Oxide ion(O 2-) electron configuration. Ground state electron configuration of oxygen is 1s 2 2s 2 2p x 2 2p y 1 2p z 1. This electron configuration shows that the last shell of oxygen has six electrons. In this case, the valence electrons of oxygen are six. The elements that have 5, 6, or 7 electrons in the last shell receive the electrons in the last shell during bond formation.
smartbook 14 Flashcards - Quizlet molecular orbitals can be formed by the combination of multiple atomic orbitals, allowing electrons to be _____ or shared between several atoms. the molecular orbital model therefore allows a better description of the bonding in _____ structure than valence bond theory, which depicts electrons as being _____ between two atoms at a time
Explain the formation of O2 molecule using molecular ... The molecular orbital energy level diagram of oxygen molecule is given as follows : Bond order 2 N b − N a = 2 8 − 4 = 2 Thus, oxygen molecule has two bonds. i.e., one is bond and one p bond.
PDF Miessler-Fischer-Tarr5e SM Ch 05 CM number of valence electrons) with O2. Therefore, NF is predicted to be paramagnetic with a bond order of 2. The populations of the bonding (8 electrons) and antibonding (4 electrons) molecular orbitals in the diagram suggest a double bond. c. The 2s, 2s *, 2p, and 2p * orbitals exhibit C v symmetry, with the NF bond axis the infinite-fold




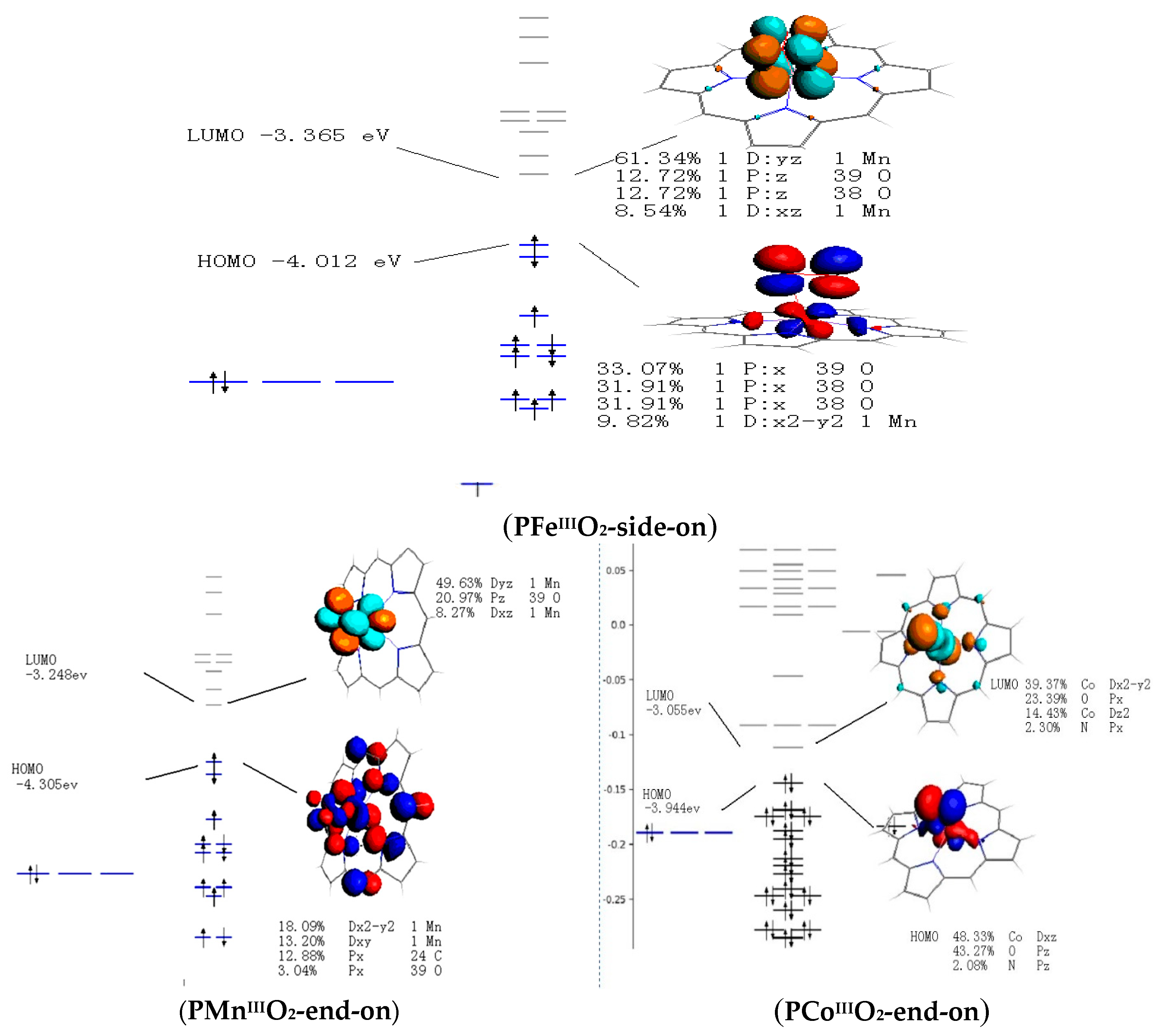
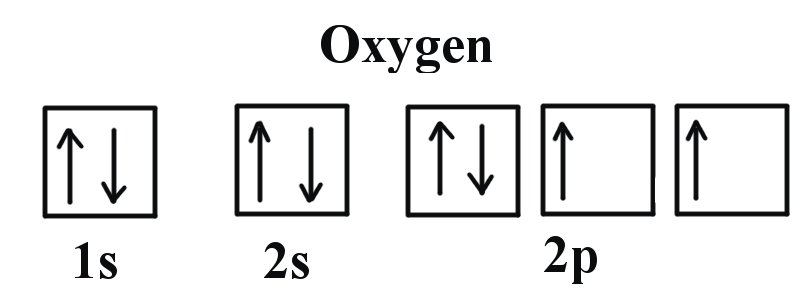



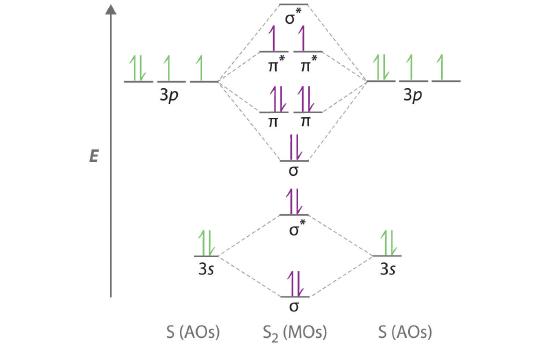









0 Response to "39 complete this valence molecular-orbital diagram for oxygen o2"
Post a Comment