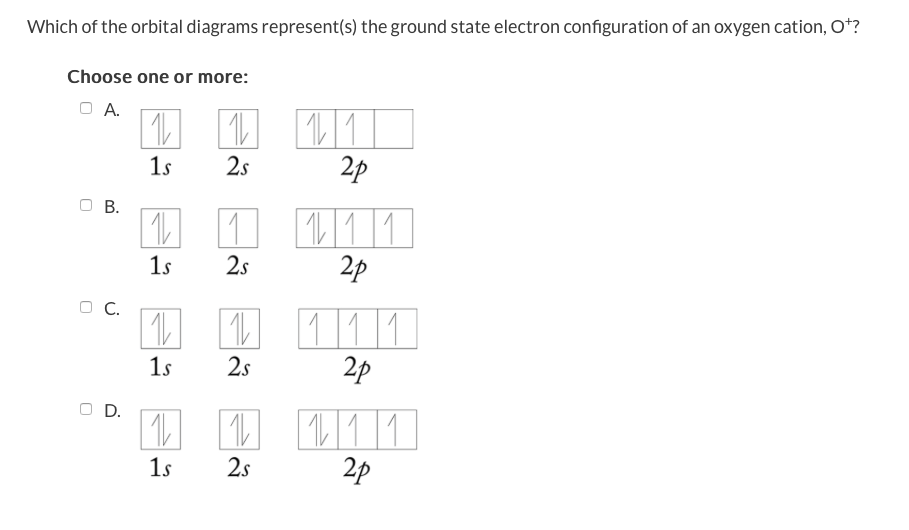
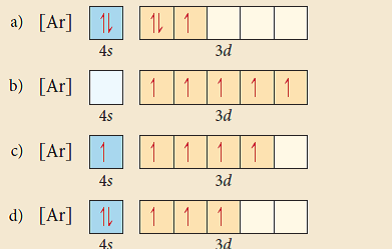
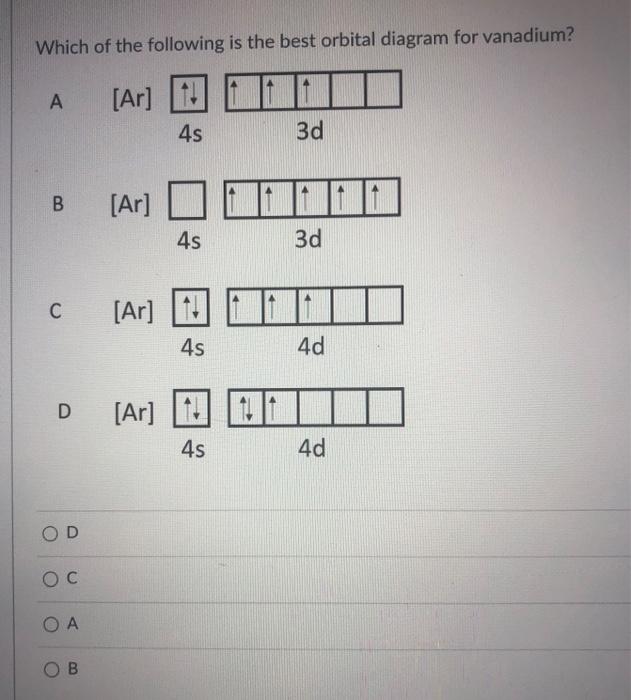
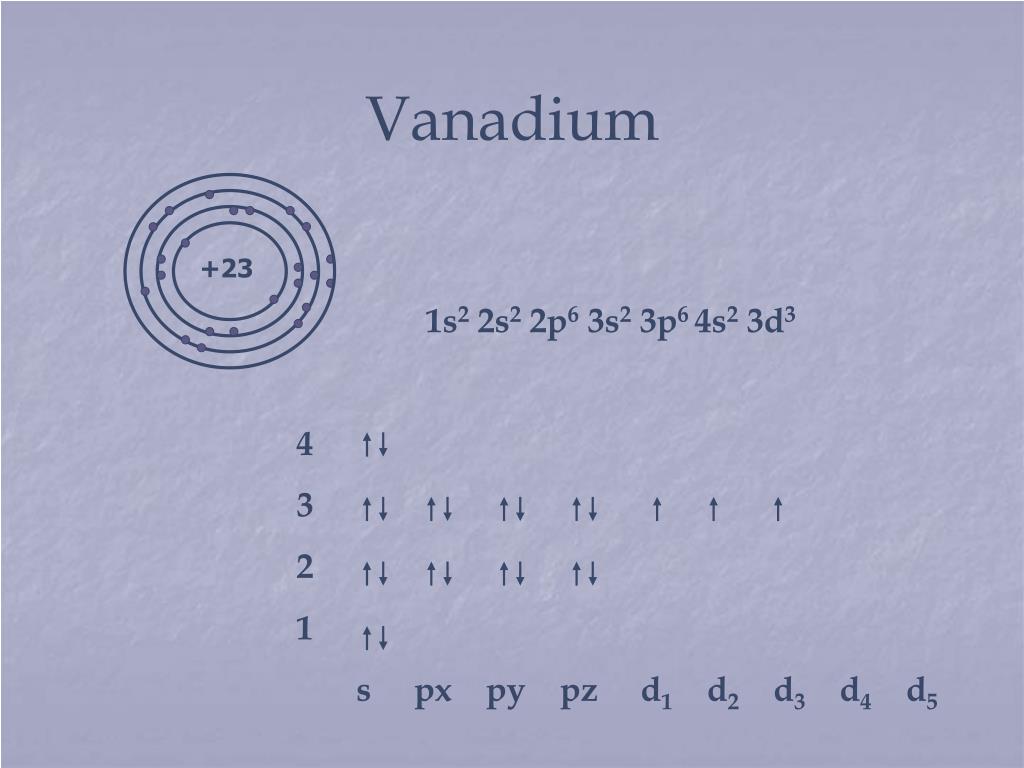
39 choose the correct orbital diagram for vanadium.
It is mixed with other metals to make very strong and durable alloys. Vanadium pentoxide (V2O5) is used as a catalyst, dye and color-fixer. Atomic Number 23 Learn more about the atomic number. Description Soft, ductile, silvery-white metal. Resistant to corrosion by moisture, air and most acids and alkalis at room temperature. Atomic Mass 50,9415 Determine the number of unpaired electrons for argon. Give the complete electron configuration of cobalt, Co, the complete or abbreviated electron configuration, of Co2+, and Co3+, and predict the number of unpaired electrons in all three. Classify each of these ions as diamagnetic or paramagnetic.Cu2+Ni2+Zn2+Co2+.
The orbital diagram, the electron configuration and the energy diagram. All three ways are useful. The next atom is helium with 2 electrons. So the second electron could go into the 1s orbital with the opposite spin of the first electron or it could go into the next orbital in the n = 2 level.
Choose the correct orbital diagram for vanadium.
4. Choose the correct orbital diagram for vanadium. a) [Ar] 1L 45 3d b) [Ar] 1 1 1 1 1 3d 4s c) [Ar] 1 1 4s 3d d) [Ar] 11 4s ; Question: 4. Choose the correct orbital diagram for vanadium. a) [Ar] 1L 45 3d b) [Ar] 1 1 1 1 1 3d 4s c) [Ar] 1 1 4s 3d d) [Ar] 11 4s l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. 1. Principal Quantum Number (n): n = 1, 2, 3, …, 8. Specifies the energy of an electron and the size of the orbital (the distance from the nucleus of the peak in a radial probability distribution plot). Chapter 9 Electrons in Atoms and the Periodic Table. 9.1 True/False Questions. 1) When the elements are arranged in order of increasing number of protons, certain sets of properties recur periodically. 2) The early scientists who developed the quantum-mechanical model were bewildered by the model and it altered our fundamental view of matter.
Choose the correct orbital diagram for vanadium.. Feb 18, 2021 · Vanadium Electron Configuration of V will be written as: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 3. Therefore many users will not be able to remember it and some will be those who will find it very difficult to write it. Therefore there is one more way that you can learn the electronic configuration and that is [Ar] 3d3 4s2. Choose the correct orbital diagram for vanadium [AR] 4s [||] [ { || } { | } { } { } { } ] ... metallic character decreases as you move to the right across a row in the periodic table and decreases as you move down a column d) metallic character increases as you move to the right across a row in the periodic table and decreases as you move down ... In the same manner of other transition metals, vanadium has added its electron to its inner shell. The configuration of reflects the addition of three transition element electrons to the third shell. Orbital Diagram. 1s Vanadium pentoxide (V2O5) is used as a catalyst, dye and color-fixer. Choose the correct orbital diagram for vanadium. 45 3d a) [Ar] 1 11 11 b) [Ar] 1|11|11 c) [Ar] 1 1111111 1) (ar 111111 Get the detailed answer: Q4. OneClass: Q4.
Apr 10, 2018 · Vanadium atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table. An orbital diagram, like those shown above, is a visual way to reconstruct the . What is the electronic configuration of vanadium (V, Z=23)?. This is what I believe to be the orbital diagram of vanadium. The Lewis electron dot diagram is a representation of valence electrons of an atom that uses dots around the symbol of element. The number of dots equals the number of valence electrons in an atom. These dots are arranged to right and left and above and below the symbol, with NO more than two dots on side. What is the Orbital Diagram For Nitrogen? When we talk about the orbital diagram, we first need to understand what exactly it means. Therefore, during exams, the student can expect questions related to this topic so it is important that the students must go through it. 37) The orbital diagram for fluorine shows 1 unpaired electron in a p orbital. 38) The correct electron configuration for magnesium is: 1s 2 2s 2 2p 6 3s 3. 39) The element manganese (symbol = Mn) has five valence electrons. 40) Bromine has 17 valence electrons. 41) Bromine has 28 core electrons.
C.) An electron in the 3p orbital. Choose the correct electron configuration for Se. A.) 1s2 2s2 2p6 3s2 3p4 B.) 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p4 C.) 1s2 2s2 2p6 3s2 3p6 4s2 4p4 D.) 1s2 2s2 2p6 3s2 3p6 4s2 3d4. B.) 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p4. What is the orbital diagram for Vanadium? [Ar] 4s= 1 up 1 down. Next orbital: 3 up arrows Orbital Diagram of All Elements (Diagrams given Below) January 1, 2022 April 10, 2021 by Admin Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. The diagram (not to scale) summarises the energies of the orbitals up to the 4p level. The oddity is the position of the 3d orbitals. They are shown at a slightly higher level than the 4s - and so it is the 4s orbital which will fill first, followed by all the 3d orbitals and then the 4p orbitals. Vanadium has oxidation states in its compounds of +5, +4, +3 and +2. This section looks at ways of changing between them. It starts with a bit of description, and then goes on to look at the reactions in terms of standard redox potentials (standard electrode potentials). Observing the changes in the lab. Reducing vanadium(V) in stages to ...
Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table.. The electron configuration for the first 10 elements. H #1s^1# He #1s^2# Li #1s^2 2s^1# Be #1s^2 2s^2# B #1s^2 2s^2 2p^1# C #1s^2 2s^2 2p^2# N #1s^2 2s^2 2p^3# O #1s^2 2s^2 2p^4# F #1s^2 2s^2 2p^5#
And in Cr and Cu the excites 1 electrons because by doing that both 4s and 3d orbital gains half filled i.e the stable configuration. So in vanadium if u excite 1 electron the d orbital will still not be half filled. But 4s orbital will be half filled. Since fully filled orbital are more stable than half filled the vanadium will have 2 ...
Orbital filling of the orbitals in an element takes place according to the following rules. • Electron occupies orbitals so as to minimize the energy of the atom; therefore, lower energy orbitals fill before higher energy orbitals. Orbitals fill in the following order: • Orbital can hold only up to two electrons each.
FREE Expert Solution We’re being asked to choose the correct orbital diagram for vanadium. For that, we first need to determine the electron configuration of Vanadium. Recall that for a neutral element, Atomic number = # of protons = # of electrons. The atomic number of Vanadium is 23 and since it’s a neutral element, this means V has 23 electrons.
Which is the correct orbital diagram for vanadium? Look at pg. 333. n=4, l=1, m1= 0, ms = 1/2 ... Write an orbital diagram for each ion and determine if the ion is diamagnetic or paramagnetic? A. V^5 + ... Choose the element with the highest first ionization energy from each pair. A. Br or Bi
The complete electron for a neutral arsenic atom is: "1s"^2"2s"^2"2p"^6"3s"^2"3p"^6"3d"^(10)"4s"^2"4p"^3 Its shorthand electron configuration is: ["Ar"]"3d"^(10)"4s"^2"4p"^3 As is the chemical symbol for the element arsenic. Its atomic number is 33, which is the number of protons in the nuclei of its atoms. In a neutral atom, the number of electrons equals the number of protons, which means ...
The electron configuration shows the distribution of electrons into subshells. This list of electron configurations of elements contains all the elements in increasing order of atomic number.. To save room, the configurations are in noble gas shorthand.This means part of the electron configuration has been replaced with the element symbol of the noble gas symbol.
Example of following the Aufbau principle, Pauli principle, and Hund's rule to construct an orbital diagram for a vanadium (Z=23) atom.
Doesn't the 5s come before the 4d? I chose [Kr]5s^2 on a quiz but it was wrong. Therefore, the ground state electron configuration for Zr 2+ is : [Kr]4d 2 5s 2. In^+1 [Kr] 5s2 4d10. 1s2, 2s2, 2p6. 1) nuclear charge and relative energy of 3d and 4s orbitals, 2) relative e-e repulsions in 3d and 4s orbitals, 3) exchange energy. Booster Classes. Need an editable periodic table to edit? Enter the ...
Give the complete electronic configuration for Mn. Choose the statement that is TRUE. *Core electrons effectively shield outer electrons from nuclear charge. *Core electrons are the easiest of all electrons to remove. *Outer electrons efficiently shield one another from nuclear charge. *Valence electrons are most difficult of all electrons to ...
Chapter 9 Electrons in Atoms and the Periodic Table. 9.1 True/False Questions. 1) When the elements are arranged in order of increasing number of protons, certain sets of properties recur periodically. 2) The early scientists who developed the quantum-mechanical model were bewildered by the model and it altered our fundamental view of matter.
l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. 1. Principal Quantum Number (n): n = 1, 2, 3, …, 8. Specifies the energy of an electron and the size of the orbital (the distance from the nucleus of the peak in a radial probability distribution plot).
4. Choose the correct orbital diagram for vanadium. a) [Ar] 1L 45 3d b) [Ar] 1 1 1 1 1 3d 4s c) [Ar] 1 1 4s 3d d) [Ar] 11 4s ; Question: 4. Choose the correct orbital diagram for vanadium. a) [Ar] 1L 45 3d b) [Ar] 1 1 1 1 1 3d 4s c) [Ar] 1 1 4s 3d d) [Ar] 11 4s





















0 Response to "39 choose the correct orbital diagram for vanadium."
Post a Comment