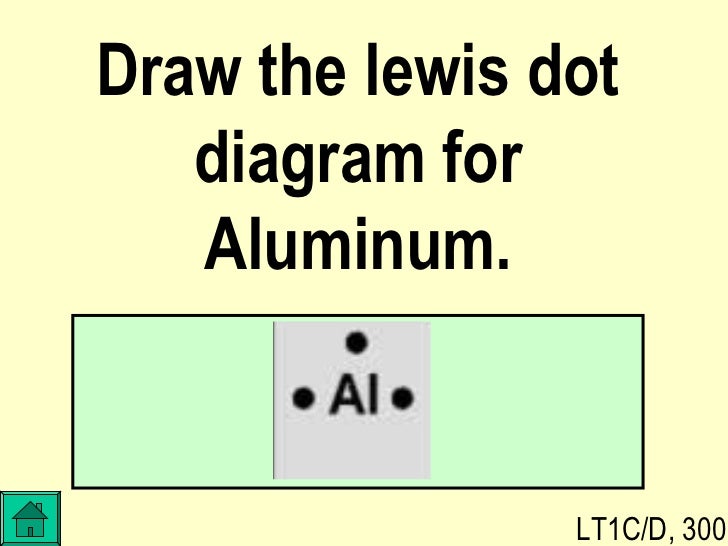
37 lewis dot diagram for aluminum
What is the Lewis electron dot diagram for each element? aluminum selenium Solution The valence electron configuration for aluminum is 3s23p1. So it would have three dots around the symbol for aluminum, two of them paired to represent the 3selectrons: The valence electron configuration for selenium is 4s24p4. In Lewis dot notation aluminum has three dots as it is group 13 and has 3 valence electrons. ... The Lewis dot diagram for neon has a pair of electrons on each side of neon symbol, Ne, for a total ...
Draw the Lewis dot diagram for aluminum. Lewis Dot Diagram: A Lewis dot diagram describes the distribution of all valence electrons present in a covalent molecule or compound, as well as a single...

Lewis dot diagram for aluminum
S o l u t i o n According to Figure 9.1, the Lewis dot symbols of Al and O are Because aluminum tends to form the cation (Al 3+) and oxygen the anion (O2−) in ionic compounds, the transfer of electrons is from Al to O. ... = 12 18 lone pairs (18x2) = 36 Total = 48 54 Example 9.9 Draw the Lewis structure for aluminum triiodide (Al I3). AlI 3 ... What is the Lewis dot structure for MG? Magnesium is element #12. To draw the Lewis electron dot diagram we picture in our minds the symbol for Mg in a box with all of its core electrons (i.e., 1s22s22p6). Then we place the valence electrons around the sides of the box with each side representing an orbital in the outermost energy level. Start studying Lewis Dot Structure Practice. In this video I will show the Lewis structure for ionic compound for aluminum oxide, Al2O3. In Lewis structures, all atoms are represented by their atomic symbols and chemical bonds by lines. Aluminum sulfide is … Step 1:The Lewis dot structure provide a picture of bonding in molecules and. ...
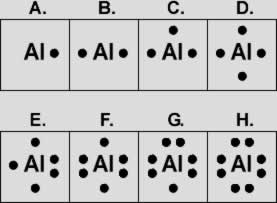
Lewis dot diagram for aluminum. Aluminum / Silicon / Potassium Xenon / Sulfur / Carbon Hydrogen / Helium (watch out!) / Bromine Selenium / Nitrogen / Barium Chlorine / Gallium / Argon. WKS 6.2 - LDS for Ions/ Typical Charges. Determine the common oxidation number (charge) for each of the following ions, and then draw their Lewis Dot Structure. Electron Dot Structures Formula Questions: 1. Give the electron dot structure for aluminum. Answer: Aluminum is in group IIIA of the periodic table therefore it has three valence electrons. The symbol for aluminum is Al which will be surrounded by three dots. 2. Give the electron dot structure of chlorine. Answer: Oct 24, 2021 · The total valence electron is available for drawing the Aluminium chloride lewis structure is 24.The hybridization of the AlCl3 molecule is Sp2 since it has a steric number equal to 3 that will form an Sp2 hybrid.AlCl3 is a nonpolar molecule because its net dipole moment is zero and charges are uniformly distributed all over the atom.In the AlCl3 lewis structure, a total of 9 lone pairs are present but no lone pair on the central atom.The molecular geometry of AlCl3 is trigonal planar with ... A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. A step-by-step explanation of how to draw the AlI3 Lewis Dot Structure.For the AlI3 structure use the periodic table to find the total number of valence elec... The Lewis dot structure for aluminum includes the symbol, "Al," and a total of three dots around the symbol. How do you draw a Lewis dot diagram for an element? Determine which atom will be the central atom of the Lewis Dot Structure. The central atom is the least most electronegative atom in the compound. Also asked, what is the Lewis dot structure for aluminum? Answer: Aluminum is in group IIIA of the periodic table therefore it has three valence electrons. The symbol for aluminum is Al which will be surrounded by three dots. 2. Subsequently, question is, how many valence electrons does aluminum oxide have? 3 valence electrons
After that I draw the Lewis dot structure for Aluminum (Al). Note: Aluminum is in Group 13 (sometimes called Group III or 3A). Since it is in Group 3 it will have 3 valence electrons. When you draw the Lewis structure for Aluminum you'll put three 'dots' or valance electrons around the element symbol (Al). Is it possible to do a lewis dot structure for a metal such as gold or aluminum? For example, would "Al Al" be correct since aluminum is content with 6 valence electrons instead of the usual 8 (octet rule). How would you do gold or copper? My son's teacher has asked him to draw a lewis dot structure for a metal. Thanks! Lewis Structure For Aluminum Fluoride, Basic Concepts of Chemical Bonding Key Terms at Chaffey, McCord Review2 Fall 2010, 31 Lewis Dot Diagram For F Wiring Diagram Database, KF Potassium fluoride Sigma Aldrich aluminum lewis dot structure . If you have any questions or good suggestions on our products and site, or if you want to know more information about our products, please write them and send to us, we will contact you within one business day.
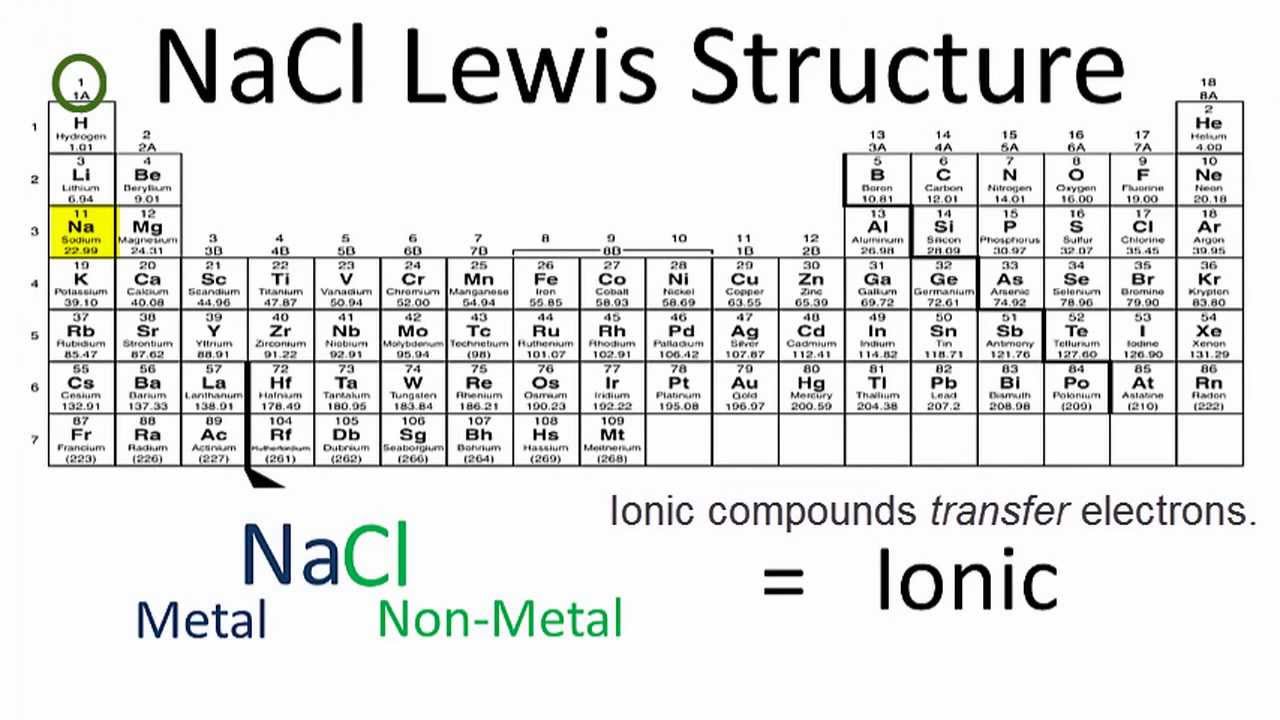
Lewis Structure of Al2O3, Aluminum Oxide By chemistnate January 30, 2021 No Comments Aluminium oxide is a solid ionic compound, made from atoms of one metal (Aluminum) that have lost three electrons each to become +3 cations, and atoms of a non-metal (oxygen) which have gained two electrons each to become -2 anions.
lewis dot structure of aluminum metal. lewis dot structure of charcoal. Group the chemical substances given below in terms of chemical bonding type that is involved in the combination of the respective atoms. You can put them in a box illustration and make a label of the containment with supporting explanations of your classification.
A step-by-step explanation of how to draw the Lewis dot structure for Al (Aluminum). I show you where Aluminum is on the periodic table and how to determine...
Hence, we have to choose the lewis diagram that has the least formal charge on each atom, Therefore, the aluminium central atom is provided with only 6 electrons instead of 8 for completing the octet shell. Summary The total valence electron is available for drawing the Aluminium chloride lewis structure is 24.
What is the Lewis electron dot diagram for each element? aluminum selenium Solution The valence electron configuration for aluminum is 3 s2 3 p1. So it would have three dots around the symbol for aluminum, two of them paired to represent the 3 s electrons: The valence electron configuration for selenium is 4 s2 4 p4.
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
draw the lewis structure for aluminum sulfide ionic compound and what chemical formula the lewis theory predicts? I do not know what to do with the 6 and 3 dot structure to combine them. Such as the AL2S AL has three dots and S has 6 dots and to balance they share to to make the 8 but I do not know where to go from here.
It is also known as the Lewis dot diagram or electron dot structure. It is the structural illustration of the position of the valence electrons, involved in the formation of a chemical bond, around the atoms inside a molecule. The dots in the proximity of the atoms represent their valence electrons.
Electron Dot Diagrams There is another model called the electron dot or Lewis diagram. This system represents an atom and its valence electrons. The electron dot diagram uses the symbol of the element to replace the nucleus and inner shell electrons. The electrons in the valence shell are shown as dots placed around the symbol.
So, if you wish to acquire all these amazing shots regarding Lewis Dot Diagram Worksheet, press save link to save these photos for your pc. They are available for obtain, if you want and want to take it, just click save symbol in the web page, and it will be directly saved to your desktop computer.} Lastly if you want to secure unique and ...
A Lewis dot diagram is a representation of an element surrounded by its valence electrons. The diagram consists of the element symbol (from the periodic table), with dots on the top, bottom, and sides representing the sand psub-levels of its valence shell. For example, aluminum has 3 valence electrons. The orbital-notation electron
Start studying Lewis Dot Structure Practice. In this video I will show the Lewis structure for ionic compound for aluminum oxide, Al2O3. In Lewis structures, all atoms are represented by their atomic symbols and chemical bonds by lines. Aluminum sulfide is … Step 1:The Lewis dot structure provide a picture of bonding in molecules and. ...
What is the Lewis dot structure for MG? Magnesium is element #12. To draw the Lewis electron dot diagram we picture in our minds the symbol for Mg in a box with all of its core electrons (i.e., 1s22s22p6). Then we place the valence electrons around the sides of the box with each side representing an orbital in the outermost energy level.
S o l u t i o n According to Figure 9.1, the Lewis dot symbols of Al and O are Because aluminum tends to form the cation (Al 3+) and oxygen the anion (O2−) in ionic compounds, the transfer of electrons is from Al to O. ... = 12 18 lone pairs (18x2) = 36 Total = 48 54 Example 9.9 Draw the Lewis structure for aluminum triiodide (Al I3). AlI 3 ...



:max_bytes(150000):strip_icc()/Thulium-58b601513df78cdcd83ccbd2.jpg)















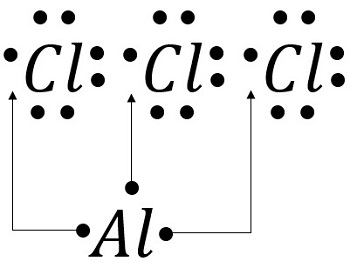
0 Response to "37 lewis dot diagram for aluminum"
Post a Comment