2 bohr diagram of fluorine
Draw Bohr diagrams of a magnesium atom bonding with fluorine atoms. Draw atoms then electron transfer and finally the ions that form. What type of bonding occurs? Draw Bohr diagrams of 2 hyrdogen atoms bonding with an oxygen atom by sharing electrons to . Sorry- Chemistry / Ionic Bonding Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8, 1: 12: Bohr model of Magnesium (Mg) 2, 8, 2: 13: Bohr model of Aluminum (Al) 2, 8, 3: 14: Bohr model of Silicon (Si) 2, 8, 4: 15: Bohr ...
A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels. Jan 18, · Aluminum, Al Bohr Diagram.
Bohr diagram of fluorine
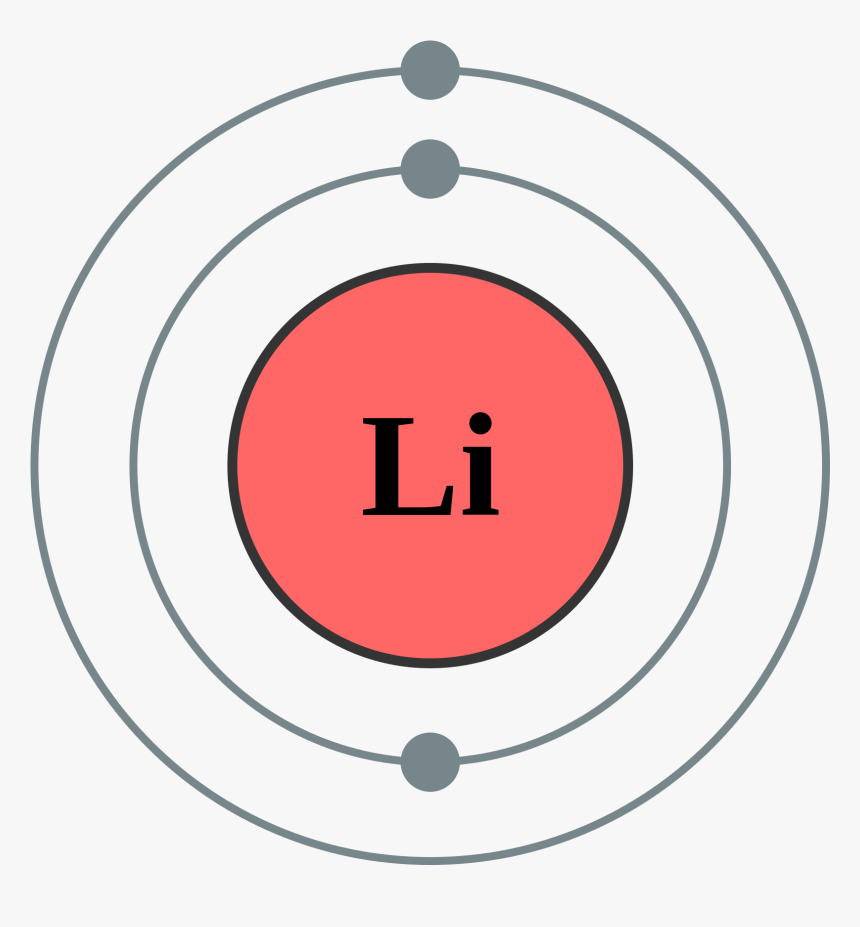
Bohr Model Diagram Cards Bohr Model Super Teacher Worksheets Homeschool Kindergarten . Figure 2 contrast the Bohr diagrams for lithium fluorine and aluminum atoms. Bohr diagram for argon. Last class we determined that the Bohr Model is a planetary model in which the For example there are 3 shells in the bohr diagram of Argon. Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He DIAGRAM OF FLUORINE ATOM album depeche mode blasphemous rumours, titeuf le film, thor le film, rio le film, peter pan le film, lol le film, le film thor, le film de justin bieber, le film 300, le film 2012, ashbahan, manual sugar cane juicer, le filet restaurant montreal, le filet de sole, kyle ashbaugh, jason ashbaugh, dennis ashbaugh, dena ashbaugh, billy ashbaugh, ovation ashbaland, mona ...
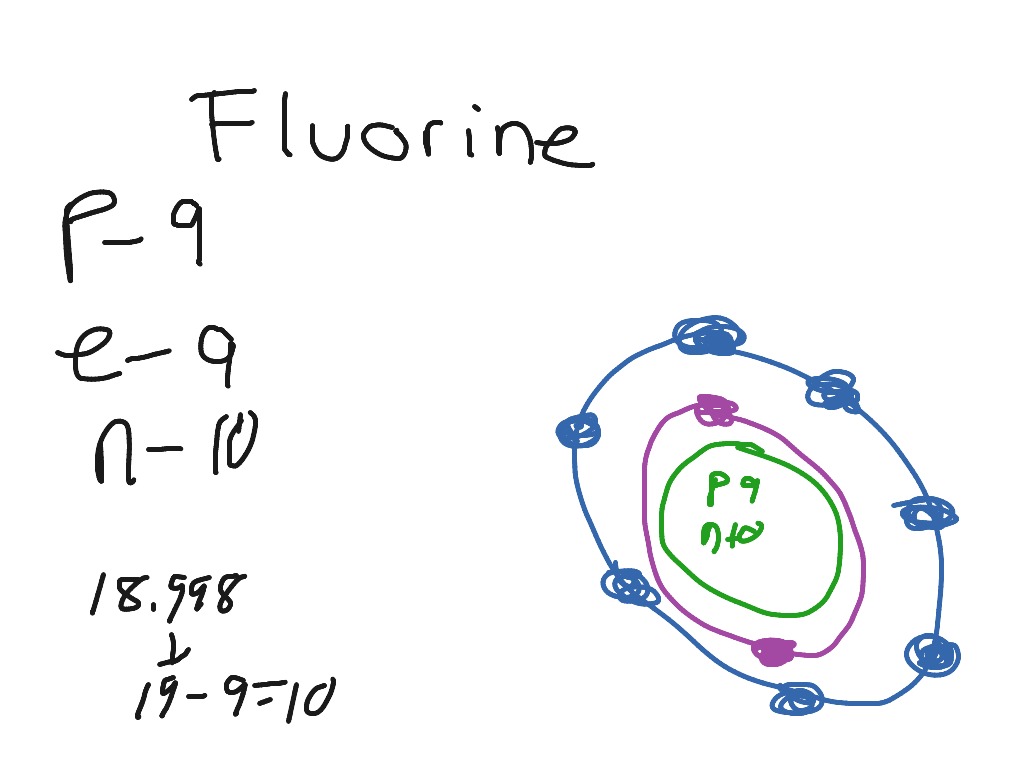
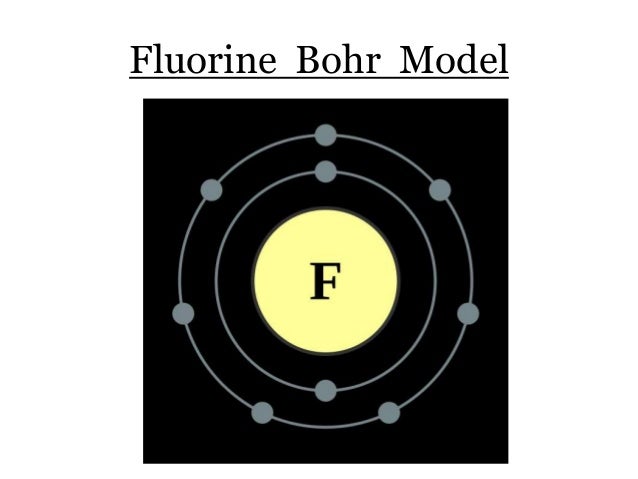
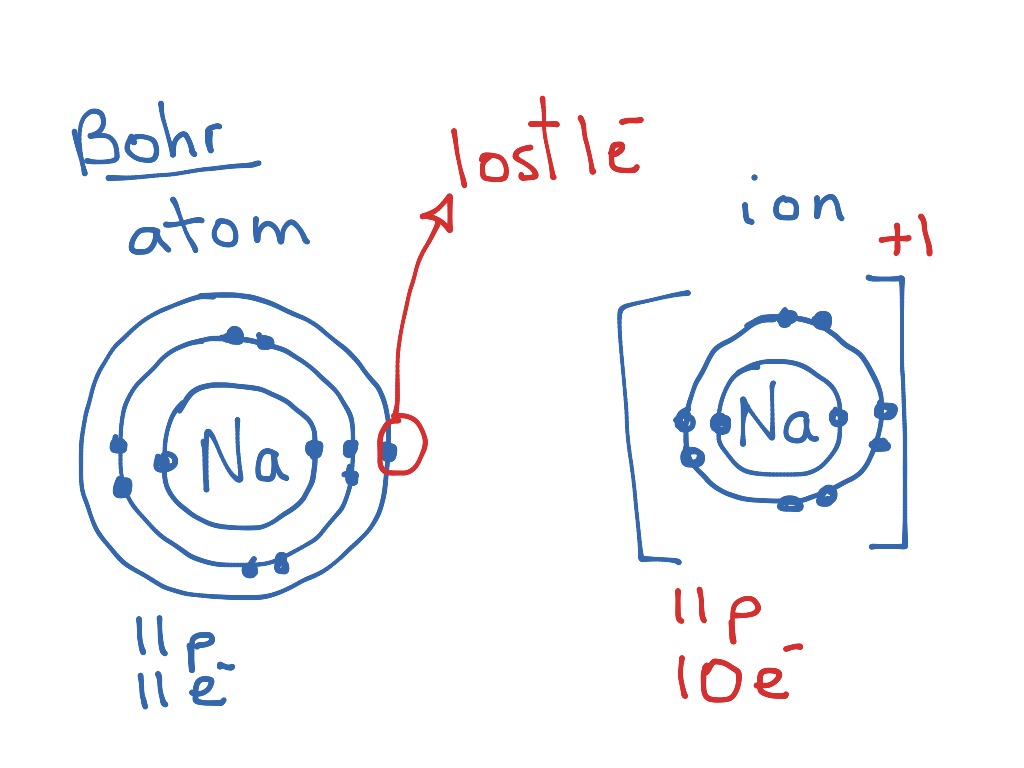
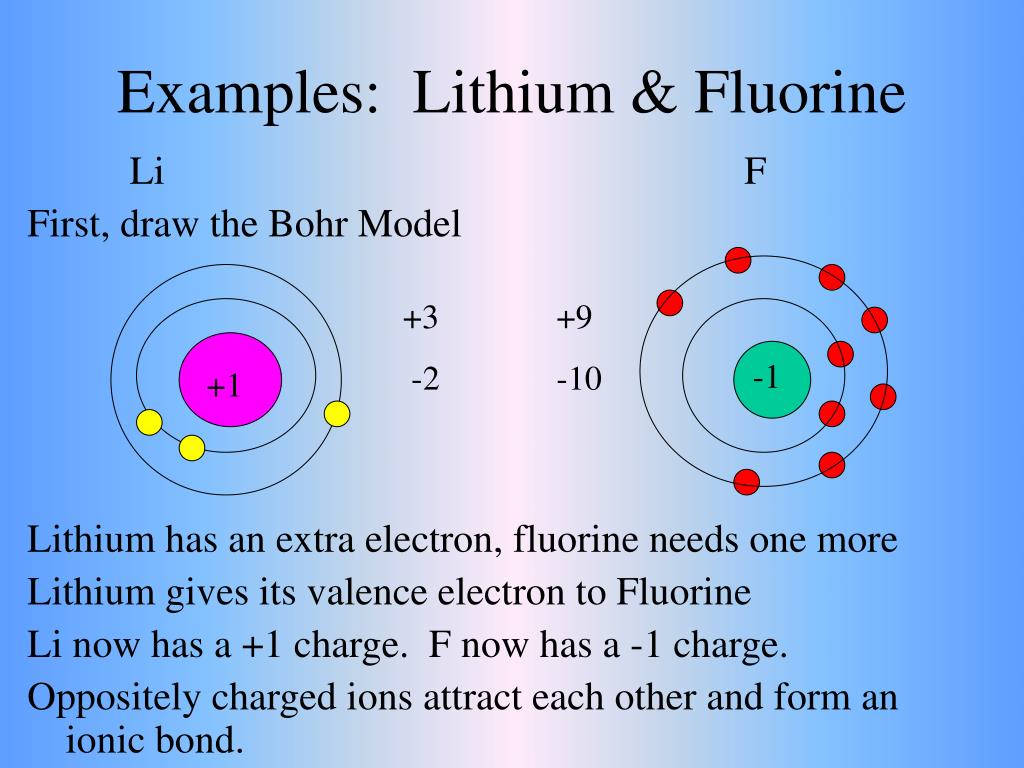
Bohr diagram of fluorine. Fluorine gains one electron to have an octet. Formation of Positive Ion: After the electron is lost by sodium, it becomes it becomes a positive ion. (11 p + 10 e- = +1). Formation of Negative Ion: After fluorine gains the electron from sodium, it becomes a negatively charged ion. (9 p + 10 e- = -1). 2. Use the table above to draw the Bohr model diagram for the following atoms and ions. Argon atom 22N Chlorine atom Chlorine ion 19 Potassium atom Potassium ion 3. What do you notice about the arrangement of electrons in the Bohr model of a neon atom, fluorine ion, and a magnesium ion? ALI e ec Levels 4. Bohr diagram for fluorine? - Answers Fluorine has an atomic number of 9. protons in the nucleus. Most fluorine around the world has 10 neutrons in the nucleus (mass number of 19). There will be an... Bohr diagrams show electrons orbiting the nucleus of an atom In the Bohr model, electrons are pictured as traveling in circles at different shells, Each element, when electrically neutral, has a number of electrons For example, the 1n shell represents the first energy level located closest to the nucleus.Now offering rare physics books for sale ...
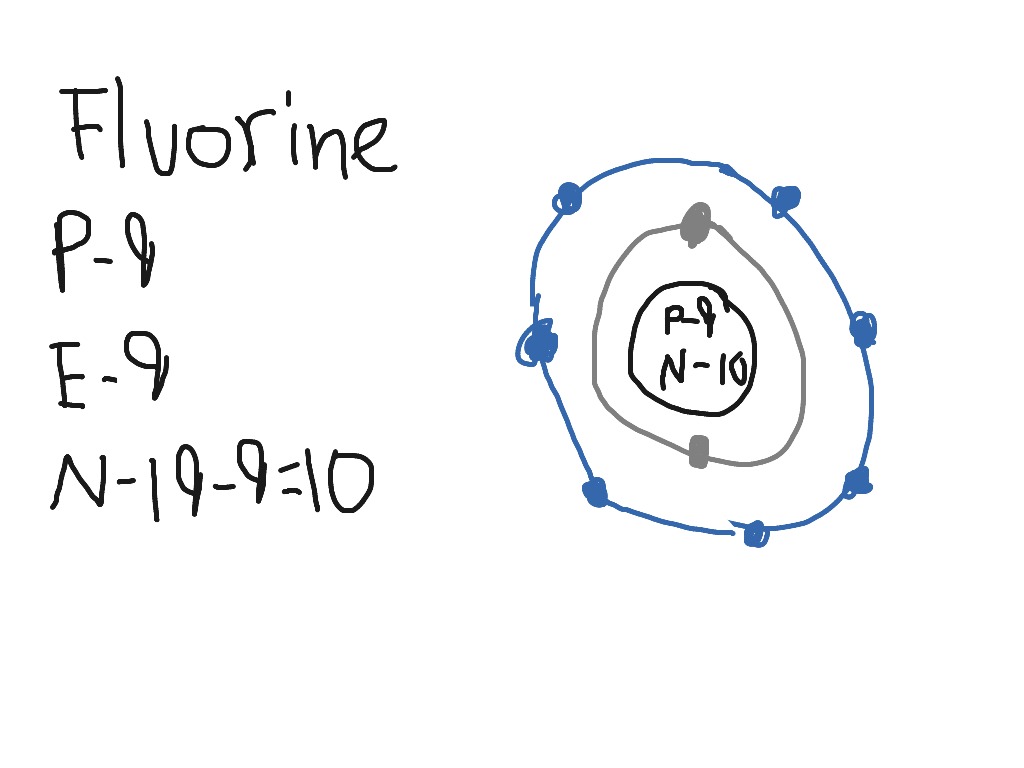
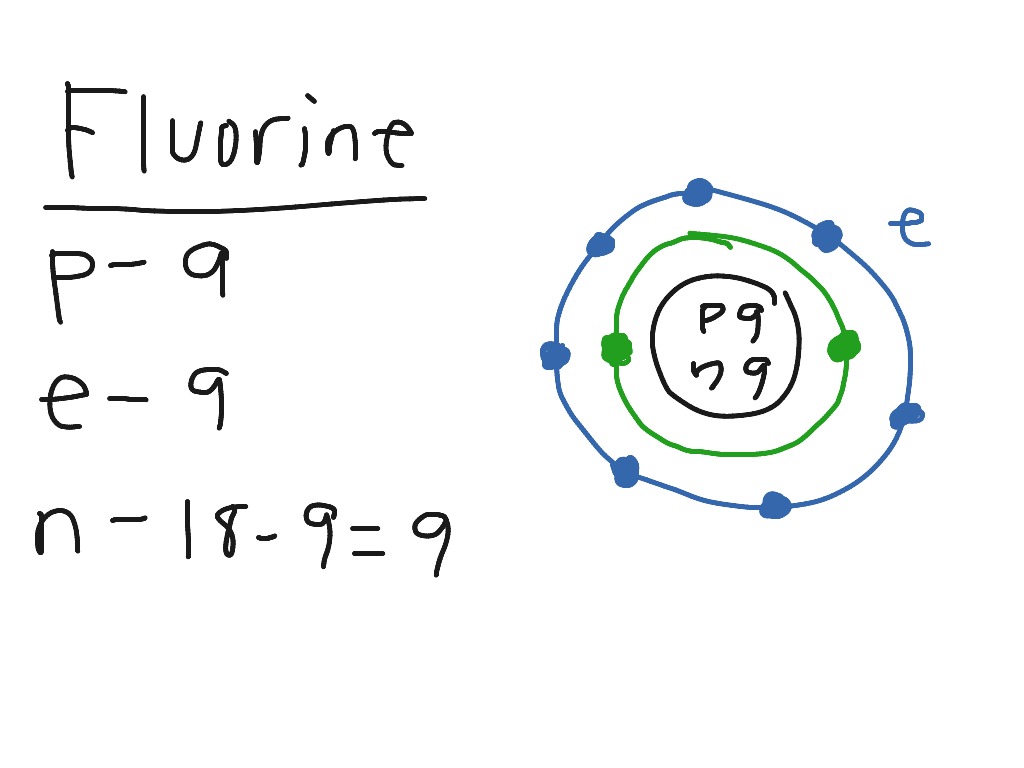
Fluorine has an atomic number of 9. This means there are 9 protons in the nucleus. Most fluorine around the world has 10 neutrons in the nucleus (mass.Figure \ (\PageIndex {2}\) contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is called the K shell, next is the L shell, next is the M shell. Fluorine has 2 electrons in its first shell and 7 in its second.Check me out: http://www.chemistnate.com This is what a fluorine looks like according to the bohr Model. Located in the middle is the nucleus. The nucleus of an atom is made of protons and neutrons ...1 answer · 1 vote: We don't know what atoms look like, because it's too small to see with out current technology, ... Magnesium has 2 electrons in its first shell, 8 in its second and 2 in its third.Check me out: http://www.chemistnate.com
Photo "Bohr model of Fluorine Atom with proton, neutron and electron. Science and chemical concept 3d illustration" can be used for personal and commercial purposes according to the conditions of the purchased Royalty-free license. The image is available for download in high resolution quality up to 10000x6670. Fluorine(F) is the 9th element in the periodic table and the first element in group-17. The standard atomic mass of fluorine is 18.998403 and its symbol is 'F'. The period of fluorine is 2 and it is a p-block element. The electron configuration of fluorine(F) and the orbital diagram is the main topic of this article. Wiring Diagram Pictures - schematron.org Draw Bohr diagrams of a magnesium atom bonding with fluorine atoms. What type of bonding occurs? Type of bond: _____ 14 . 4. Complete the following table. Note that the name of a NON-METALLIC ion ends in - IDE while the name for a METALLIC ion uses the full name of the metal. ION NAME . ION ...
Rutherford-Bohr diagram Lewis notation If an element has more than four valence electrons, they are doubled to form pairs of dots around the element. For example, neon is represented by eight valence electrons. Rutherford-Bohr diagram Lewis notation SAMPLE QUESTIONS: 1) Draw a Lewis structure for each of the following elements: Potassium
The Bohr Model.draw a Bohr-Rutherford diagram for nitrogen. draw a Bohr-Rutherford diagram for oxygen. draw a Bohr-Rutherford diagram for fluorine. a Bohr-Rutherford diagram is used to show the numbers and locations of protons, neutrons, and electrons in an atom. step 1.
Lewis Dot Diagram- The sodium transfers one electron to the fluorine so they can both become stable. Bohr-Rutherford Diagram: Sodium is the cation as it has a positive charge after losing one electron, while fluoride is the anion as it has a negative charge after gaining one electron. The two atoms attract, as they have opposite charges.
Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we'll show a sample Bohr diagram for hydrogen. H —Hydrogen. 1 proton. 1 electron. 0 neutrons
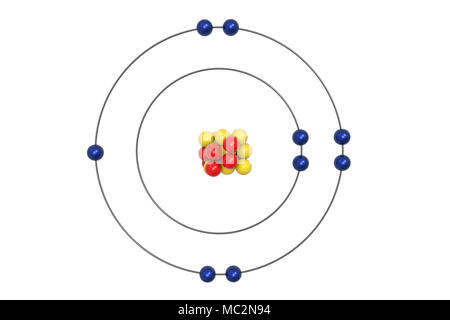
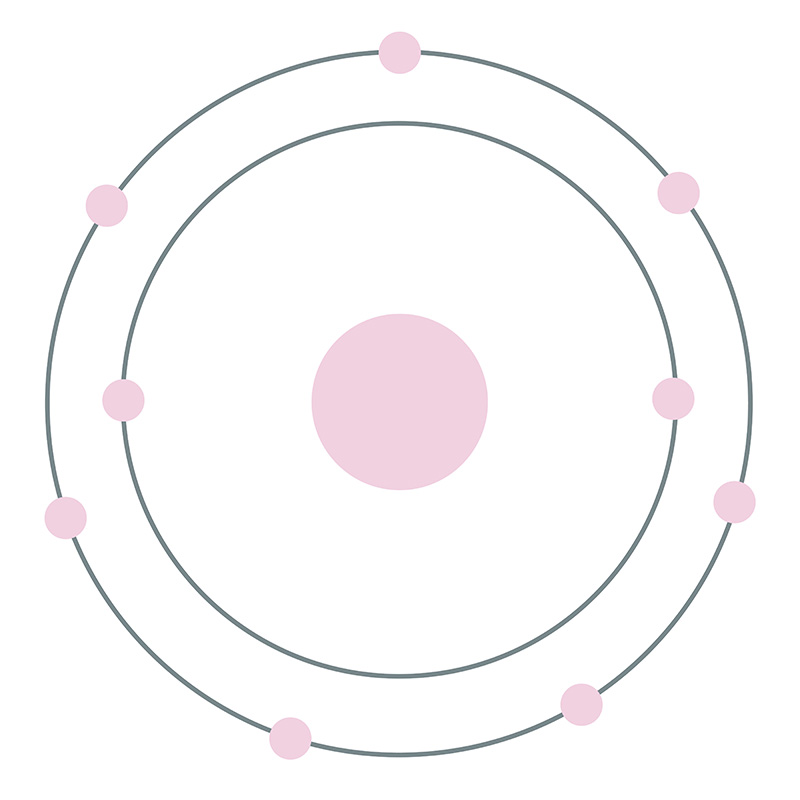
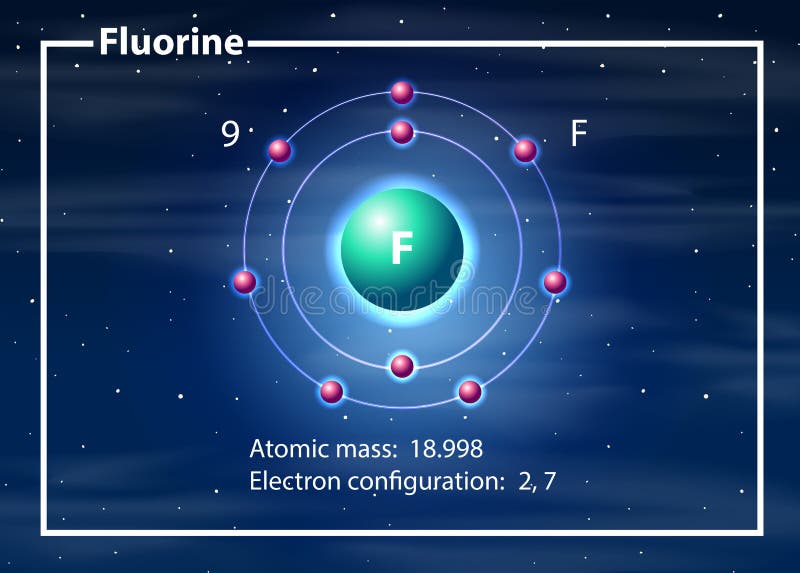
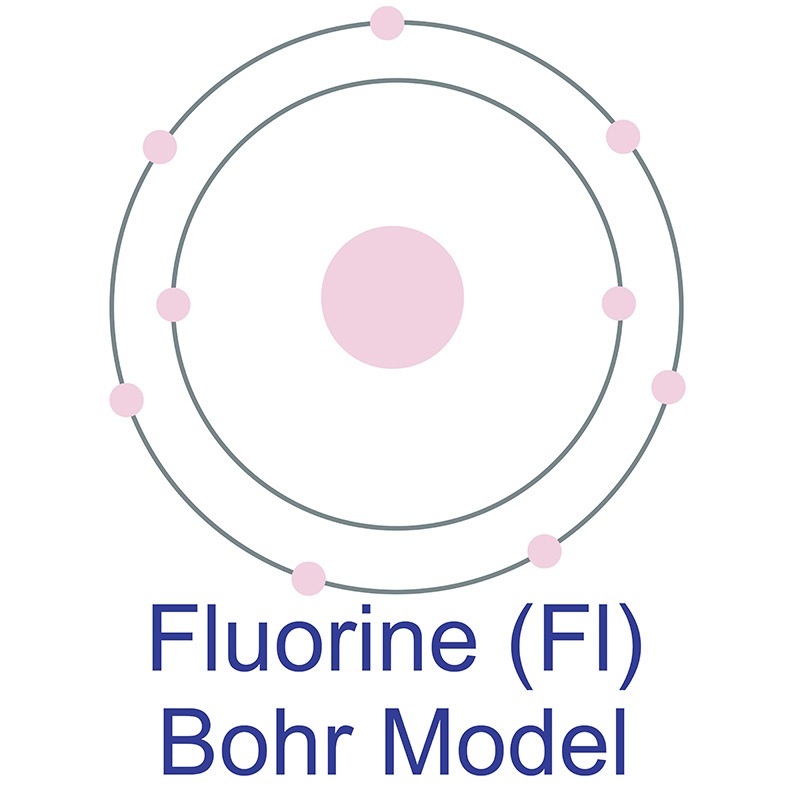
According to Bohr's model of the atom, electrons orbit about the nucleus much like the way planets orbit the sun. The diagram below shows the Bohr model for fluorine. The nucleus of fluorine has 9 protons. Surrounding the nucleus of fluorine is 9 electrons. The electrons arrange themselves in 3 orbits: In the first orbit, there are 2 electrons.
Steps to draw the Bohr Model of the atom. 1. Find the number of protons, electrons, and neutrons. The number of protons for the atom is the same as its atomic number. So, the atomic number for Fluorine is 9, hence, the number of protons in the Fluorine atom is also 9. Now, to determine the number of neutrons in an atom, use this formula.
Different energy levels are associated with the different orbits. The diagram below shows the Bohr model for fluorine. The nucleus of fluorine has 9 protons. Surrounding the nucleus of fluorine is 9 electrons. The electrons arrange themselves in 3 orbits: In the first orbit, there are 2 electrons. In the second orbit, there are 7 electrons.
A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in 1913. The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels. ... A fluorine atom has nine protons and nine electrons, so it ...
Write the Lewis diagrams of the following elements, representative of the groups. Note that every member of the group will have the _____ Lewis diagram. Lithium Beryllium Boron Carbon. Nitrogen Oxygen Fluorine Neon. Bohr Diagrams of the Atom. Atoms can be represented by . Bohr. diagrams.
Fluorine 9p ton 2e Fe- Group 17 , VMA F or7 Fluorine atom Bohr Diagram Lewis symbol Difference between Bohr diagram & Lewis symbol Bohr diagrams can become crowded . Low's dot diagrams the Lewis bot structure is a bit diff from the bohr Model . It only shows the element symbol and it's outer must electron shell.
Niels Bohr (1913): -studied the light produced when atoms. were excited by heat or electricity. Rutherford's model couldn't explain why. unique colours were obtained by atoms of. different elements. Bohr proposed that electrons are in orbits &. when excited jump to a higher orbit. When.
Bohr's diagram of Fluorine has only two electron shells (K and L), the inner shell is K-shell and the outermost shell is L-shell. Hence, the electrons found in the L-shell of the Fluorine atom are its valence electrons because it is the outermost shell that also called the valence shell.
Fluorine has seven of eight possible electrons in its outermost energy level, which is energy level II. It would be more stable if it had one more electron because this would fill its outermost energy level. How do Bohr diagrams work? Bohr Diagrams. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around ...
DIAGRAM OF FLUORINE ATOM album depeche mode blasphemous rumours, titeuf le film, thor le film, rio le film, peter pan le film, lol le film, le film thor, le film de justin bieber, le film 300, le film 2012, ashbahan, manual sugar cane juicer, le filet restaurant montreal, le filet de sole, kyle ashbaugh, jason ashbaugh, dennis ashbaugh, dena ashbaugh, billy ashbaugh, ovation ashbaland, mona ...
Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He
Bohr Model Diagram Cards Bohr Model Super Teacher Worksheets Homeschool Kindergarten . Figure 2 contrast the Bohr diagrams for lithium fluorine and aluminum atoms. Bohr diagram for argon. Last class we determined that the Bohr Model is a planetary model in which the For example there are 3 shells in the bohr diagram of Argon.












0 Response to "2 bohr diagram of fluorine"
Post a Comment