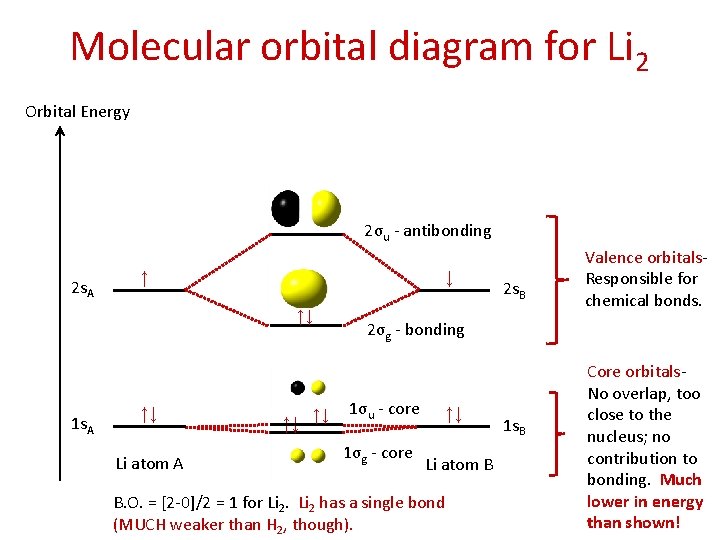
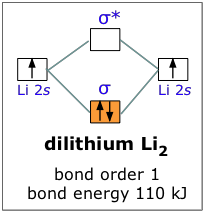
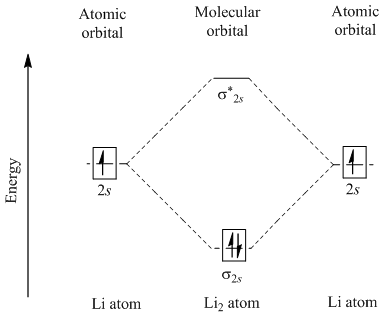
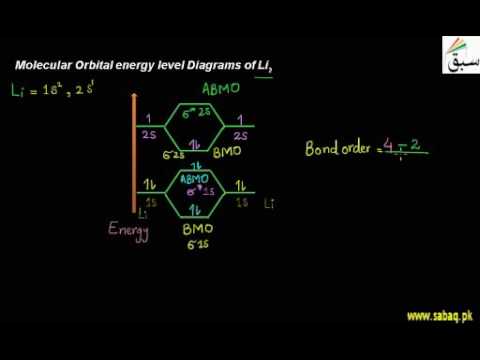
42 molecular orbital diagram for li2
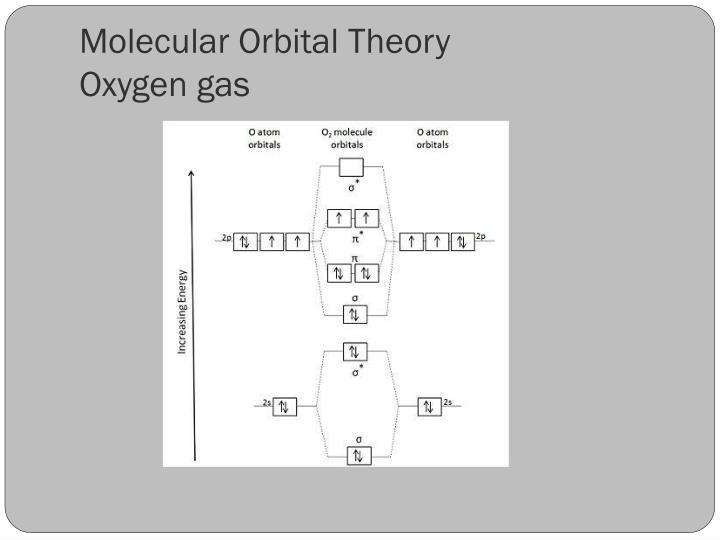
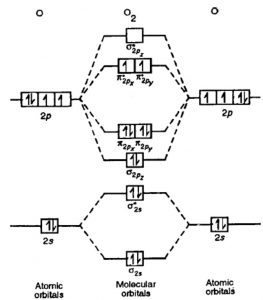
Draw the M.O diagram for oxygen molecule and calculate its bond order and show that O2 is paramagnetic. asked Dec 17, 2020 in Chemical Bonding by Panna01 (47.2k points) ... Draw and explain the molecular orbital diagram of carbon molecule. asked Dec 22, 2020 in Chemical Bonding by Aashi01 (13.0k points) B) Li2 is stable and diamagnetic, but Be2 is unstable. C) Be2 is stable and diamagnetic, but Li2 is unstable. D) Be2 is stable and paramagnetic, but Li2 is unstable. 32) Given that O2 is paramagnetic and has a bond order of 2, and its highest occupied molecular orbital is antibonding, what would be the expected bond orders for O22- and O22+? A ...
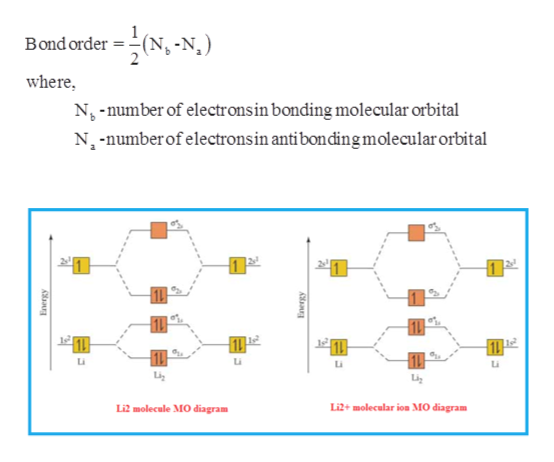
Q. Draw the molecular orbital (MO) energy diagram for Li2+. Solved • Nov 12, 2018 MO Theory: Homonuclear Diatomic Molecules Q. Some species consisting of just two oxygen atoms are the oxygen molecule, O 2; the peroxide ion, O22−; the superoxide ion, O 2−; and the dioxygenyl...
Molecular orbital diagram for li2
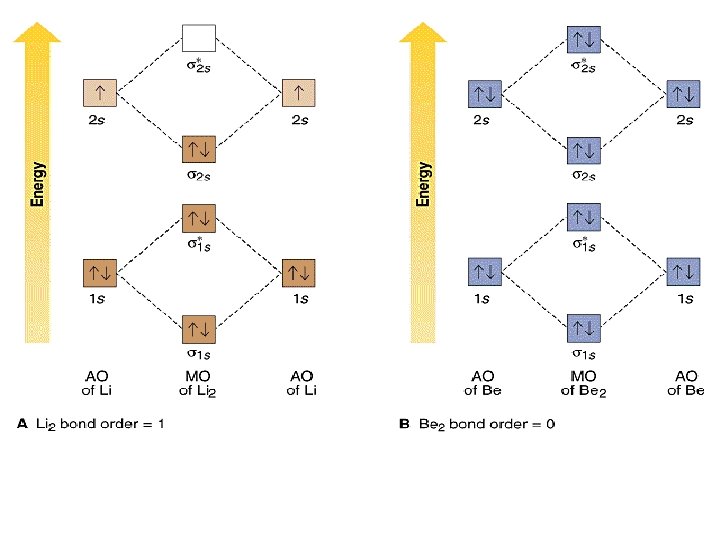
(b) Using your MO diagram, is Li2 paramagnetic or diamagnetic? (c) Does the bond order predicted by MO theory agree with VB theory from a Lewis electron dot diagram? 8 13.16 Diatomic neon (Ne,) has a dissociative ground state and simple molecular orbital theory pre dicts bond order zero. Excited states of diatomic neon may have nonzero bond order. The lowest energy unoccupied molecular orbital is 2pσ, so that is where the extra electron will be added. The electron configuration of the neutral C2 molecule is -- I'll use the notation given to you in the diagram. C2:(1sσ)2(1s* σ)2(2sσ)2(2s* σ)2(2pπ)4.The electron configuration of the C− 2 ion will be. The Molecular Orbital Diagram For C2 ^ 2-Question: The Molecular Orbital Diagram ... 3:26This video shows that the pi framework and sigma framework of ethylene are distinct orbital sets.18 Jan 2010 · Uploaded by jeffrey Moore Interactions between Ethylene Molecular Orbital s and Metal d Orbital s. could not be loaded. Script error: Forbidden is not defined. Ethylene is capable of acting as a ligand as the C=C π bond can donate electron density to an empty metal d orbital ...
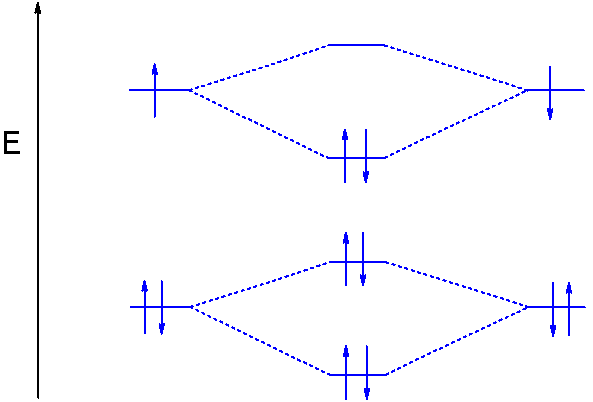
Molecular orbital diagram for li2. Figure 9.7. 3: Molecular Orbital Energy-Level Diagrams for Diatomic Molecules with Only 1 s Atomic Orbitals. (a) The H 2+ ion, (b) the He 2+ ion, and (c) the He 2 molecule are shown here. Figure 9.7. 3 a shows the energy-level diagram for the H 2+ ion, which contains two protons and only one electron. Use the mo diagrams to calculate the bond order for li2+ and li2−. According to molecule orbital concept predict the link order for C2. = n/2 = 2/2 = 1. O. 06eV in every bond. Ii) Molecules and ions having total Qualcuno Ha Comprato Cialis online no the electrons within the variety (2-6): In such situation Bond stimulate = ns 4- n i / 2 ;. Molecular Orbital Diagrams. This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H 2 +. Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as is the case for atomic ... 14+ H2 Molecular Orbital Diagram. A molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of. The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals.
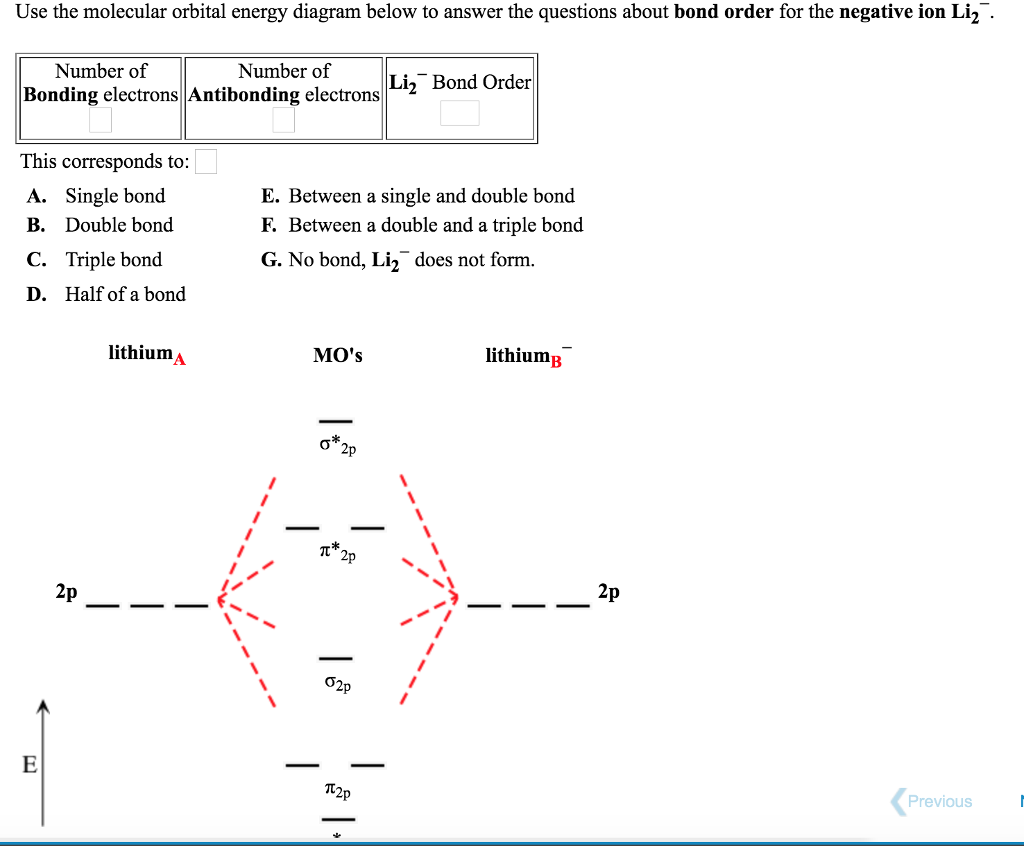
Use the molecular orbital energy diagram below to answer the questions about bond order for the positive ion Li2+. Number of Bonding electrons Number of Antibonding electrons Li2+ Bond Order This corresponds to: ..... A. Single bond E. Between a... Molecular orbital diagram for he2+. A molecular orbital explicitly describes the spatial distribution of a single electron orbital s, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative ... The molecular orbital diagrams of , and are drawn in the attached image. There is no unpaired electron present in the MO diagram of and all the electrons are paired up so it is diamagnetic in nature. There is one unpaired electron present in the MO diagram of and therefore it is paramagnetic in nature. Q. Draw a molecular orbital energy diagram for ClF. (Assume that the σp orbitals are lower in energy than the π orbitals.) What is the bond order in ClF? ... Q. Part AUse the drawing of the MO energy diagram to predict the bond order of Li2+.Express the bond order as an integer or fraction.Part BUse the dra... Solved • Oct 19, 2020
Molecular orbital energy level diagram of N 2 molecule • Bond order = (8 2)/2 = 3 (N ≡ N) • Absence of unpaired electrons showed that N 2 molecule is diamagnetic. Atomic orbitals are inherent property of an atom. by subtraction or addition of wave functions of atomic orbitals, The above equation forms two molecular orbitals. Question: Use Molecular Orbital Theory To Determine Whether He2 Or He2+ Is More Stable. These properties can be explained by the molecular orbital diagram of BN". The bond order of two suggests that the oxygen molecule is stable. Correct option (a) O-2. Diamagnetic Metals + properties give you a broad overview of these metals from multiple angels. F2 Molecular Orbital (MO) Diagram. As per molecular orbital (MO) theory, all the constituent atoms in a molecule contribute to the formation of molecular orbitals. These MOs are a linear combination of the atomic orbitals. Thus, the electrons in a molecule are not individually assigned to atomic orbitals but to molecular orbitals. Let us have a ... When we make the molecular orbital energy level diagram of f2 molecule then, we will get this configuration: 1σs 2, 1σ*s 2, 2σs 2, 2σ* 2, σ2pz 2, π2p x 2, π2p y 2, πp x * 2, π2p y * 2. From this electronic configuration, we can see that there are a total of ten bonding molecular orbitals and eight antibonding molecular orbitals.
Li2 Molecular Orbital Diagram Wiring Site Resource. The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine.
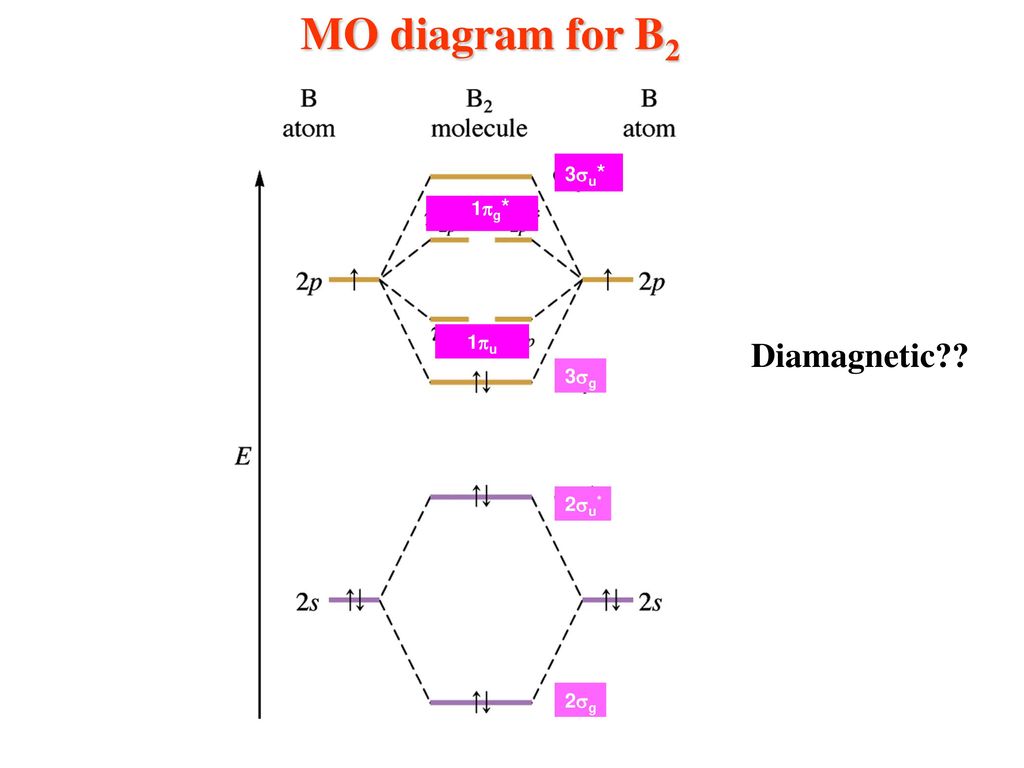
When comparing Be2 and H2: I. This is the molecular orbital diagram for the homonuclear diatomic Be2+, . which ion below has a noble gas electron configuration? The electronic configuration of Beryllium is 1s 2 2s 2. BF 3 3. B2 molecule is formed by the overlap of atomic orbitals of both boron atoms. Answer: Al 3+: 1s 2 2s 2 2p 6 Given : S 2-Sulfur will gain two electrons when it forms an ion ...
Li2 Molecular Orbital Diagram Google Search Chemistry How To Rationalise With Mo Theory That Co Is A Two Electron Figure 5 From Molecular Orbitals Of The Oxocarbons Co N N Molecular Orbital Diagram Of Co Brainly In Draw The Molecular Orbital Energy Diagram For Co To Predict ...
Does Li2 have a bond order of 2? It's going to equal to ½ times the number of electrons in the bonding molecular orbital minus the number of electrons in the antibonding molecular orbital. So 2 - 1 is 1, and then ½ * 1 is just ½ or 0.5 so bond order for this molecule would be ½ and it's paramagnetic because it has one unpaired electron.
The bond order can be interpreted from MO diagram s using the following for mula: `" Bond Order" = 1/2 [(" Bond ing "e^-)-("Anti bond ing " e^-)]` One half the difference between the number of electrons present in the bond ing and the anti-bond ing orbitals is bond order Bond order (B.O) =1/2(Nb−Na) Bond order of H2− To tal number of ...
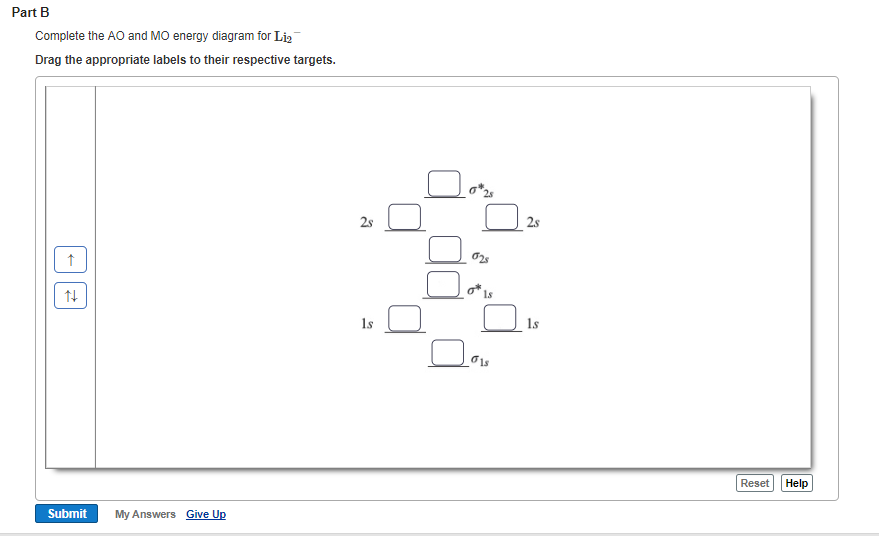
Since the bond order of Li 2 is higher than Li 2 + and Li 2 - . Therefore ,Li2 has stronger single covalent bonding . Thus lithium molecule (Li2) is more stable. Li 2 + is more stable as compared to Li 2 - :. Li 2 + and Li 2 - ions have the same bond order ( 0.5) .. But Li 2 - has more electrons in higher energy antibonding molecular orbital as compared to Li 2 + .
How to draw Molecular Orbital Diagram of Li2 ,Li 2+ , Li2 - | Simplest Trick - Chemistry By anumsunum on August 20, 2021 • ( Leave a comment ) Also Watch Molecular orbital diagram of O2 , O2 +2 , 02 - 2 ( in Urdu / Hindi)
Answer (1 of 2): 1 My approach is different. I use the quantum numbers as the quantitized, hemispherical coordinates. That provides the shells build: * in 2 hemispheres in one dimension (so 2x - always even count) * in the tightest configuration in the remaining two dimensions, a circle, so ...
Molecular Orbital Diagram of N2. Molecular orbitals exist in molecules where each molecule has its electron configuration in terms of a sigma bond and pi bond. According to molecular orbital theory, it tells about magnetic nature, stability order, and the number of bonds in a molecule.
11+ Li2 Molecular Orbital Diagram. This chemistry video tutorial provides a basic introduction into molecular orbital theory. (a) the diagram for h2, he2, li2, be2, b2, c2, and n2. Relationship between electronic configuration and molecular behaviour. Number of electrons in c2 molecule = 12.
Answer (1 of 2): As Carbon has atomic number =6 and mass number =12 .So it has 6 electrons and protons while it's electronic configuration is 1s2 ,2s2,2p2. In C2 , two atoms of carbon bonded together to form a molecule. On the basis of molecular orbital theory (MOT), it has electronic configurat...
3:26This video shows that the pi framework and sigma framework of ethylene are distinct orbital sets.18 Jan 2010 · Uploaded by jeffrey Moore Interactions between Ethylene Molecular Orbital s and Metal d Orbital s. could not be loaded. Script error: Forbidden is not defined. Ethylene is capable of acting as a ligand as the C=C π bond can donate electron density to an empty metal d orbital ...
The lowest energy unoccupied molecular orbital is 2pσ, so that is where the extra electron will be added. The electron configuration of the neutral C2 molecule is -- I'll use the notation given to you in the diagram. C2:(1sσ)2(1s* σ)2(2sσ)2(2s* σ)2(2pπ)4.The electron configuration of the C− 2 ion will be. The Molecular Orbital Diagram For C2 ^ 2-Question: The Molecular Orbital Diagram ...
(b) Using your MO diagram, is Li2 paramagnetic or diamagnetic? (c) Does the bond order predicted by MO theory agree with VB theory from a Lewis electron dot diagram? 8 13.16 Diatomic neon (Ne,) has a dissociative ground state and simple molecular orbital theory pre dicts bond order zero. Excited states of diatomic neon may have nonzero bond order.



















0 Response to "42 molecular orbital diagram for li2"
Post a Comment