40 solid liquid phase diagram
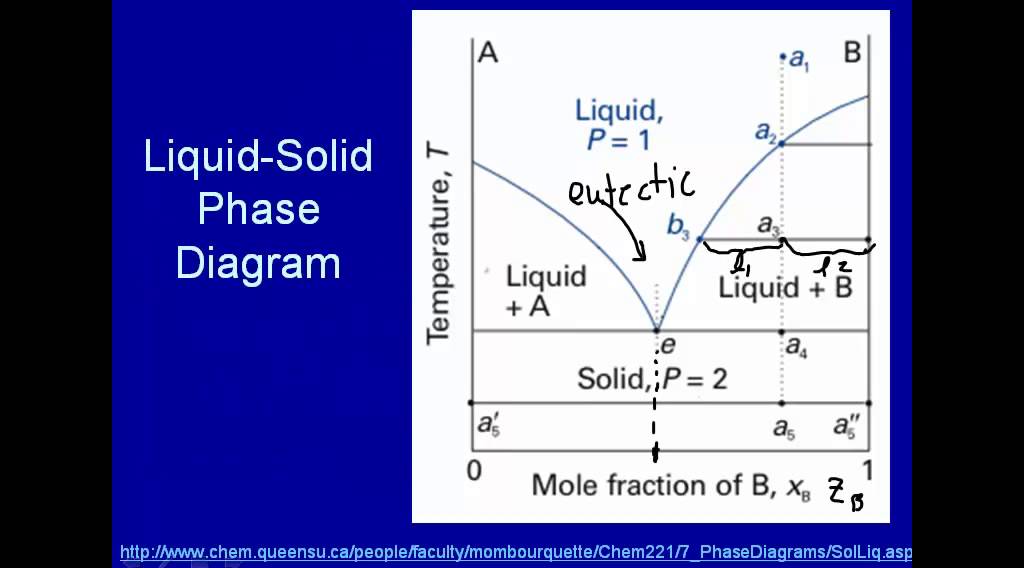
5.9 Liquid-solid phase diagrams Key points 1. At the eutectic composition the liquid phase solidifies without change of composition 2. The phase equilibria of binary systems in which the components react may also be summarized by a phase diagram 3. In some cases, a solid compound does not survive melting
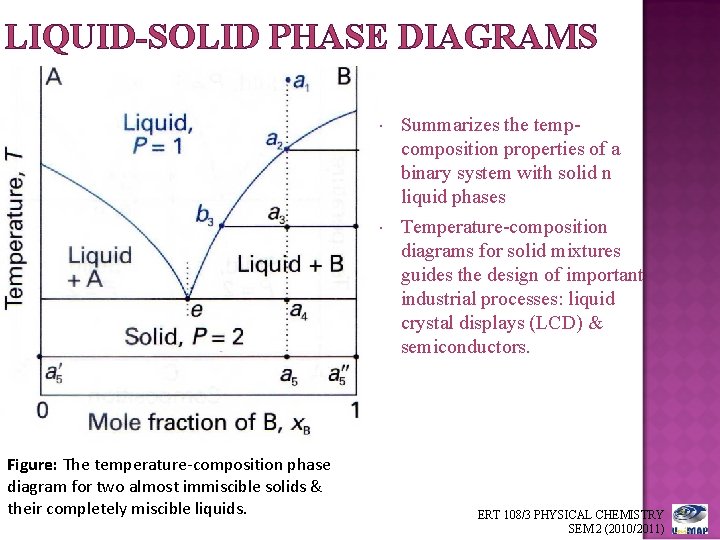
• Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash (Ed.), ASM International, Materials Park, OH (1991). • 2 phases: L (liquid) α (FCC solid solution) • 3 phase fields: L L + α α 0 20 40 60 80 100 wt% Ni 1000 1100 1200 1300 1400 1500 1600 ...
The labelled areas in the phase diagram. These areas all show what you would see if you had a particular mixture of salt and water at a given temperature. For example, if the temperature was below -21.1°C, you would always see a mixture of solid salt and ice. There would never be any liquid whatever proportions of salt and water you had.

Solid liquid phase diagram
SOLID - LIQUID PHASE DIAGRAM Important: bring a formatted 3.5" floppy diskette/USB flash drive for this laboratory - you will need it to save your data files! Introduction The relation of cooling curves to phase diagrams form the basis of "thermal analysis", an important technique for constructing phase diagrams .
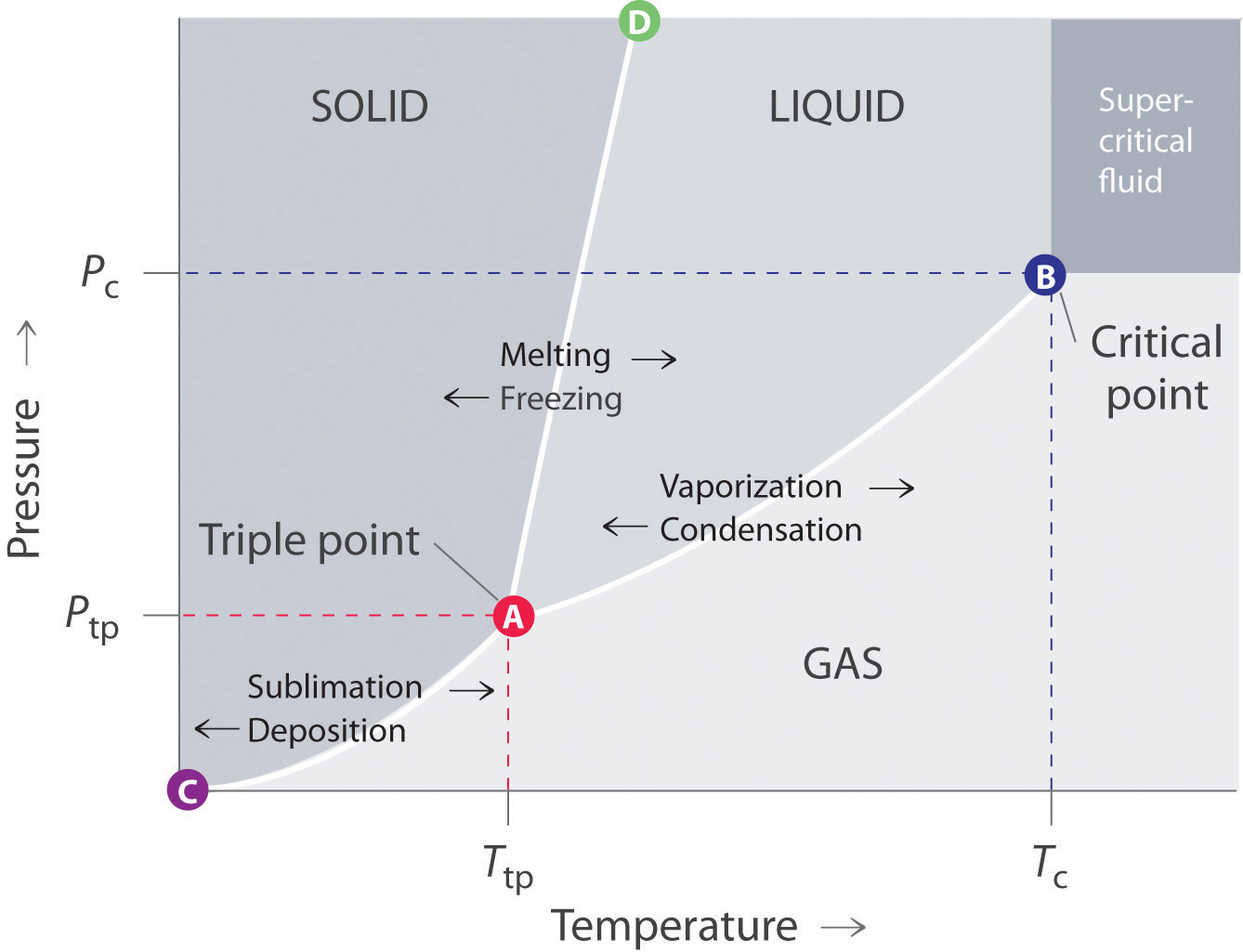
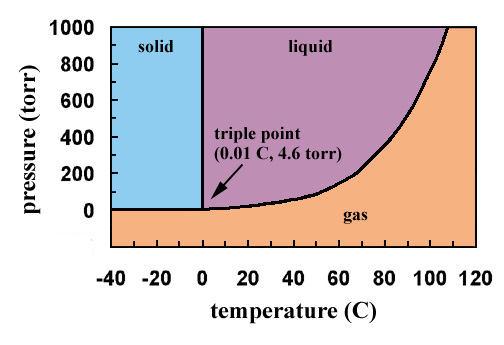
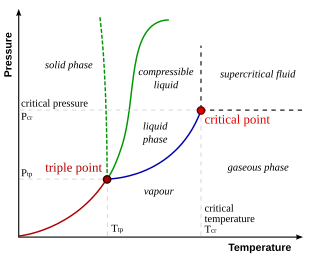
Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating that carbon dioxide cannot exist as a liquid ...
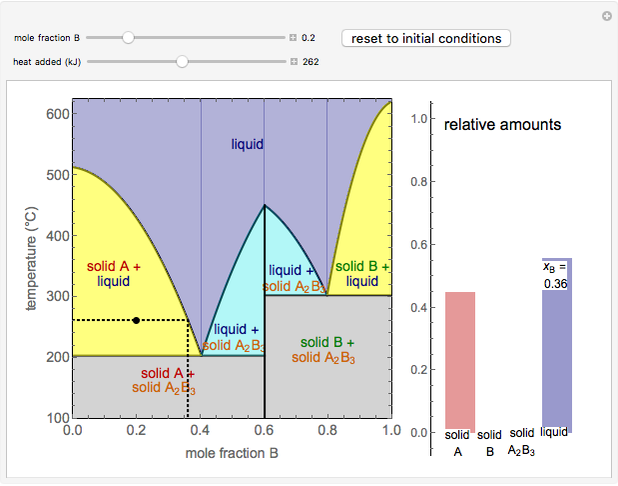
Solid-liquid equilibrium and solid-solid-liquid equilibrium are both represented in the phase diagram. Mixtures of pure solids are immiscible. The relative amounts of the four possible phases, given the temperature and mole fraction of (represented by the black point in the - - diagram), are shown in the bar graph to the right of the diagram.
Solid liquid phase diagram.
If it is decreasing, the liquid phase is denser, if it is increasing, the solid phase is denser. For example, the phase diagram of water has a negative solid-liquid line; the liquid phase of water is denser. The densest phase exists at the highest pressure and lowest temperature. This comes back to looking at the top left corner of the graph. Share
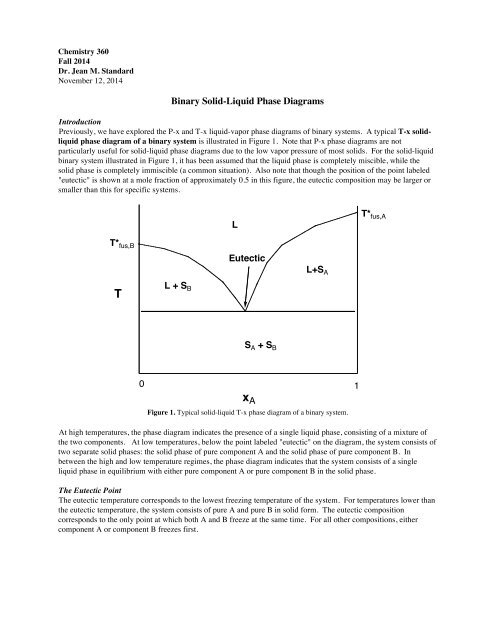
Expt. 5: Binary Phase Diagram CHEM 366 V-1 Binary Solid-Liquid Phase Diagram Introduction The substances that we encounter in the material world are hardly ever pure chemical compounds but rather mixtures of two or more such compounds. The individual substances in such a mixture
Phase diagrams give the state of a substance (solid, liquid, gas) given a specific set of conditions such as temperature and pressure. Below is an example of a simple phase diagram for water. 1 It is well known that water exists as a solid at or below its freezing point of 0 °C.
A phase diagram for an organic compound could include mesophases, which are intermediate phases between a solid and a liquid. Mesophases are of particular interest for liquid crystal technology. While phase diagrams look simple at first glance, they contain a wealth of information concerning the material for those who learn to read them.
The solid/liquid solution phase diagram can be quite simple in some cases and quite complicated in others. Let's begin by looking at a simple two-component phase diagram with components that are fully miscible in both the liquid and solid phase. The diagram to the right shows a simple two-component A,B system solid-liquid phase diagram.
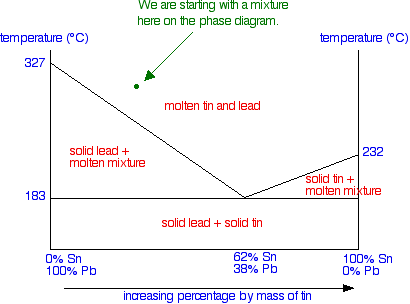
SOLID-LIQUID PHASE DIAGRAMS: TIN AND LEAD This page explains the relationship between the cooling curves for liquid mixtures of tin and lead, and the resulting phase diagram. It also offers a simple introduction to the idea of a eutectic mixture.
Phase Diagrams. The figure below shows an example of a phase diagram, which summarizes the effect of temperature and pressure on a substance in a closed container. Every point in this diagram represents a possible combination of temperature and pressure for the system. The diagram is divided into three areas, which represent the solid, liquid ...
Isomorphous system -complete solid solubility of the two components (both in the liquid and solid phases). Binary Isomorphous Systems (I) Three phase region can be identified on the phase diagram: Liquid (L) , solid + liquid (α +L), solid (α ) Liquidus line separates liquid from liquid + solid Solidusline separates solid from liquid + solid α+ L α
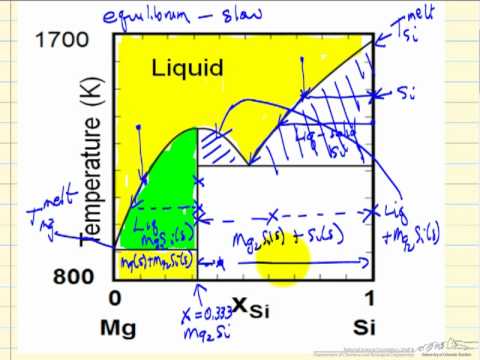
Describes the regions of the T-x phase diagram for two miscible metals, describes the behavior as a liquid is cooled to form a solid, and discusses why the t...
Solid-liquid phase diagrams show the phase relationships in mixtures of two or more components and are very important in understanding the behavior of mixtures in metallurgy, material science and geology.
In general, a liquidus is the locus of points in a phase diagram repre- senting the temperatures at which alloys of the various compositions of begin freeze on cooling or finish melting heating; a solidus is the locus of points representing temperatures at which the various alloys finish freezing on cooling or begin melting heating.
Solid-Liquid Phase Diagram Using Di erential Scanning Calorimetry Objective The purpose of this laboratory is to use a modern di erential scanning calorimeter to measure the liquid-solid phase diagram (T-˜) and associated thermodynamic parameters (eutectic point, H, C p, S, etc..) of a binary compound or elemental system. Introduction
solid lumps within a liquid mixture of A and B. These two phase partially solid regions are marked on the phase diagram. TUTORIAL ON BINARY PHASE DIAGRAMS Binary Phase Diagrams: Simple Eutectic Systems Eutectic solidification: Consider a mixture of elements A
Solid-liquid Phase Diagram . Ref: P.A. Giguere. "Complements au Nouveau Traite de C. himie Minerale - No. 4 - Peroxyde d'Hydrogene et Polyoxydes d'Hydrogene" Paris, Masson 1975 (181 p).
The solid solution phase diagram explains the behavior of chemical solid solution series, such as the transition from high temperature, calcium-rich plagioclase to low temperature sodium-rich plagioclase, or the transition from high temperature magnesium-rich to low temperature iron-rich crystals in ferromagnesium minerals (e.g. olivine, pyroxene).
The system enters the two phase region labeled 'liquid + B'. Pure solid B begins to come out of solution and the remaining liquid becomes richer in A. (2) a 2 ® a 3. More of the solid forms, and the relative amounts of the solid and liquid (which are in equilibrium) are given by the lever rule.
For most substances, the solid-liquid phase boundary (or fusion curve) in the phase diagram has a positive slope so that the melting point increases with pressure. This is true whenever the solid phase is denser than the liquid phase.
The liquid system begins to solidify when the temperature cools to the liquidus temperature. The solidus is represented by a line on a phase diagram that separates a solid phase from a solid + liquid phase region. The system is not completely solid until it cools below the solidus temperature.
Phase diagrams are combined plots of three pressure-temperature equilibrium curves: solid-liquid, liquid-gas, and solid-gas. These curves represent the relationships between phase-transition temperatures and pressures.
12.1.3 Solid-Liquid Equilibrium Phase Diagrams Solid-liquid equilibrium data are obtained experimentally by cooling a liquid mixture of known composition and recording the temperature continuously as a function of time.7 A break point in this curve indicates the formation of a solid phase.
•The phase diagram plots relative concentrations of A and B along the X-axis, and temperature along the Y-axis. The eutectic point is the point where the liquid phase borders directly on the solid α + β phase; it represents the minimum melting temperature of any possible A B alloy.
liquid-vapor plateau and solid-vapor plateau. In this experiment, the phase diagram is shown for the solid-liquid equilibrium point, and varies from 100% composition of naphthalene to 100% composition of biphenyl. Further examples of this particular type of phase diagram are in the ChE101 lab manual and in the preliminary report questions.
Applies the lever rule to a solid-liquid mixture to determine the fraction of each phase in equilibrium and explains the basis for the lever rule. Made by fa...
![PDF] Binary Solid-Liquid Phase Diagrams of Selected Organic ...](https://d3i71xaburhd42.cloudfront.net/09a535d7b5c50d0b01b218090add9194c69b8824/1-Figure1-1.png)

:max_bytes(150000):strip_icc()/phase_diagram_generic-56a12a1b5f9b58b7d0bca817.png)





![ZOpt] Consider the temperature-composition phase … - ITProSpt](https://cdn.numerade.com/ask_images/284a27cf69b94a9a9701c93fff8d4413.jpg)







![Solid-liquid Phase Diagram [PDF|TXT]](https://html.pdfcookie.com/02/2020/01/07/7rv3839wreld/bg1.jpg)

![Phase transformations and phase diagrams [SubsTech]](https://www.substech.com/dokuwiki/lib/exe/fetch.php?w=&h=&cache=cache&media=complete_solubility.png)




0 Response to "40 solid liquid phase diagram"
Post a Comment