39 the diagram below represents a spontaneous reaction
Nov 10, 2021 · The diagram below represents a spontaneous reaction (δg. The spontaneous redox reaction in a voltaic cell has _____ A) a negative value of Ecell and a negative value of ΔG. B) a positive value of Ecell and a positive value of ΔG. C) a negative value of Ecell and a positive value of ΔG. D) a positive value of Ecell and a negative value of ΔG. 15. The potential energy diagram below represents a reaction. Which arrow represents the activation energy of the forward reaction? 1) A 3) C 2) B 4) D 16. The potential energy diagram below shows the reaction X + Y → Z. When a catalyst is added to the reaction, it will change the value of 1) 1 and 2 3) 2 and 3 2) 1 and 3 4) 3 and 4 17.
The figure below represents the spontaneous reaction of H2 (shaded spheres) with O2 (unshaded spheres) to produce gaseous H2O. ΔH = -, ΔS = -,ΔG = - ... According to the diagram above, the forward reaction is. spontaneous at d, at equilibrium at e, and nonspontaneous at f.
The diagram below represents a spontaneous reaction
Solved Label The Diagram Below With The Correct Values Fo. ... The Diagram Represents A Spontaneous Reaction Use The. Click Images to Large View The Diagram Represents A Spontaneous Reaction Use The. Solved Unit 7 Energy Worksheet 6 Quantitative Energy Pro. Problem: The diagram represents a spontaneous reaction. Use the diagram to answer the questions below. Is the reaction endothermic or exothermic? What is the activation energy of the reaction? FREE Expert Solution. Recall that an energy diagram is usually read from left to right. temperature. The products of t his reaction are liquid lead and water vapor. As the reaction proceeds, water vapor and excess hydrogen gas leave the glass tube. The diagram and balanced equation below represent this reaction. Write a balanced half-reaction equation for the reduction of the ions in this reaction.
The diagram below represents a spontaneous reaction. Base your answers on the information and diagram below, which represent the changes in potential energy that occur during the given reaction. Given the reaction: A + B --> C. a) Does the diagram illustrate an exothermic or an endothermic reaction? State one reason, in terms of energy, to support your answer. The diagram represents a spontaneous reaction. To find the measure of k simply plug in the value for x into 3x2 and you have your answer. What is the activation energy of the reaction. Use the diagram to answer the questions below. 3x2 x 15 and solve for x. Consider the diagram below. 14base your answer to the following question on the diagram ... 54.The spontaneous decay of an atom is called 55.The diagram below represents a nuclear reaction in which a neutron bombards a heavy nucleus. Afission Bfusion Calpha decay Dbeta decay Which type of reaction does the diagram illustrate? Acombustion Breduction Cnuclear fission Dnuclear fusion 56.In which type of reaction do two lighter nuclei combine The Diagram Represents A Spontaneous Reaction. Use the Diagram to Answer the Questions Below. - the Diagram Represents A Spontaneous Reaction. Use the Diagram to Answer the Questions Below. , solved the Diagram Shown Shows the Reaction Profile
D)It undergoes a spontaneous redox reaction. 9.Which statement describes one characteristic of an operating electrolytic cell? 10.Base your answer to the following question on the diagram below which represents the electroplating of a metal fork with Ag(s). A)Ag+ + NO3- ® AgNO3 B)AgNO3 ® Ag+ + NO3- C)Ag+ + e- ® Ag(s) D)Ag(s) ® Ag+ + e- The spontaneous redox reaction in a voltaic cell has _____ A) a negative value of Ecell and a negative value of ΔG. B) a positive value of Ecell and a positive value of ΔG. C) a negative value of Ecell and a positive value of ΔG. D) a positive value of Ecell and a negative value of ΔG. E) a positive value of Ecell and a value of zero for ΔG. The diagram below represents a spontaneous reaction (deltaG degree < 0). Drag the labels to the correct bins. uncatalyzed rx'n Delta G degree standard free E of activation products reactants catalyzed rx?n. Question: The diagram below represents a spontaneous reaction (deltaG degree < 0). 23.The diagram below represents a nuclear reaction in which a neutron bombards a heavy nucleus. Which type of reaction does the diagram illustrate? 1)fission 2)fusion 3)deposition 4)evaporation 24.Given the diagram representing a reaction: Which type of change is represented? 1) 2) 3) 4)
The diagram below represents a spontaneous reaction (deltaG degree < 0). Drag the labels to the correct bins. uncatalyzed rx'n Delta G degree standard free E of activation products reactants catalyzed rx?n 2789184 home questions sciencemath chemistry chemistry others the diagram represents a spontaneous reaction. The diagram represents a spontaneous reaction use the diagram to answer the questions below. B the endpoint occurs when equal moles of substances react. Is the reaction endothermic or exothermic. C)It is non-spontaneous and produces an electric current. D)It is non-spontaneous and requires an electric current. (1)Which statement describes the redox reaction that occurs when an object is electroplated? Base your answers to questions 2 and 3 on the diagram below which represents the electroplating of a metal fork with Ag(s). A)Ag+ + e ... A spontaneous reaction is characterized as having a negative value of the change in Gibb's free energy. Thus, the free energy at the end of the reaction must be less than that at the start, to make it negative. With that being said, the red curve represents the spontaneous reaction, while the blue curve is for the non-spontaneous reaction. search.
) t is non-spontaneous and requires an electri current. D) It is non-spontaneous and produces an electric current. A) anode C salt bridge B) cathode D) external 6. Given the balanced ionic equation representing the reaction in an operating voltaic cell: zn(s) + Cu2+(aq) Zn2+(aq) + cu(s) The flow of electrons through the external circuit in
Use the following potential energy diagram to answer the questions below. The diagram represents a spontaneous reaction use the diagram to answer the questions below. Kj b determine the activation energy for the reverse reaction. Note that just because a reaction is called spontaneous does not necessarily mean that the reaction will happen ...
The diagram represents a spontaneous reaction. Use the diagram to answer the questions below. Is the reaction endothermic or exothermic? endothermic exothermic What is the activation energy of the reaction? Question: The diagram represents a spontaneous reaction. Use the diagram to answer the questions below. Is the reaction endothermic or ...
The diagram below represents a spontaneous reaction δg institution. Chem1101 2014 j 12 june 2014 the diagram below represents this reaction involves an increase in the number of moles of gas so δs will be positive as δh δg tδs this means that δh δg for the other reactions there is a decrease in the number of moles of has so δs is ...
Nov 09, 2021 · 14 Dec 2020 — The diagram below represents a spontaneous reaction. Drag the labels to the correct bins. Is the reaction endo the rmic or exo the rmic? The reaction will only be allowed if the total entropy change of the universe is zero or positive. This is reflected in a negative ΔG, and the reaction is called an exergonic process.
From the values of delta H and delta S, predict which of the following reactions would be spontaneous at 28 degree C: reaction A: delta H = 10.5 kJ/mol, delte S = 30.0 J/K. mol: spontaneous nonspontaneous impossible to tell reaction B: delta H = 1.8 kJ/mol, delta S = -113 J/K middot mol, spontaneous nonspontaneous impossible to tell If either ...
According to this diagram, (starts at middle, swoops down, then to the top) ΔG° is positive and is equal to a - b. Consider the reaction 2A(g) ⇌⇌ A2(g). The following pictures represent two possible initial states and the equilibrium state of the system. ... The figure below represents the spontaneous reaction of H2 (shaded spheres) with ...
reaction below 2 Cr(s) + 3 Cu2+(aq) ® 2 Cr3+(aq) + 3 Cu(s) ... The diagram below represents a chemical cell. A) a cathode B) an anode C)a salt bridge D) an external path for electrons In order for the cell to operate, it should be provided with. Regents review Electrochemistry(redox)
potential energy diagram below, which represents the reaction: A + B ® C + energy. A) 1 B) 2 C) 3 D) 4 Which numbered interval will change with the addition ... 36.A reaction will be spontaneous if it results in products that have A) negative and the reaction is spontaneous B) negative and the reaction is not spontaneous ...
The diagram represents a spontaneous reaction. Use the diagram to answer the questions below. a. Is the reaction endothermic or exothermic? b. What is the activation energy of the reaction?
11.Given the potential energy diagram representing a reversible reaction: The activation energy for the reverse reaction is represented by A) 1 and 3 B) 3 and 4 C)2 and 3 D) 1 and 2 12.The potential energy diagram below shows the reaction X + Y Z. When a catalyst is added to the reaction, it will change the value of A) A B)B C) C D) D
temperature. The products of t his reaction are liquid lead and water vapor. As the reaction proceeds, water vapor and excess hydrogen gas leave the glass tube. The diagram and balanced equation below represent this reaction. Write a balanced half-reaction equation for the reduction of the ions in this reaction.
Problem: The diagram represents a spontaneous reaction. Use the diagram to answer the questions below. Is the reaction endothermic or exothermic? What is the activation energy of the reaction? FREE Expert Solution. Recall that an energy diagram is usually read from left to right.
Solved Label The Diagram Below With The Correct Values Fo. ... The Diagram Represents A Spontaneous Reaction Use The. Click Images to Large View The Diagram Represents A Spontaneous Reaction Use The. Solved Unit 7 Energy Worksheet 6 Quantitative Energy Pro.
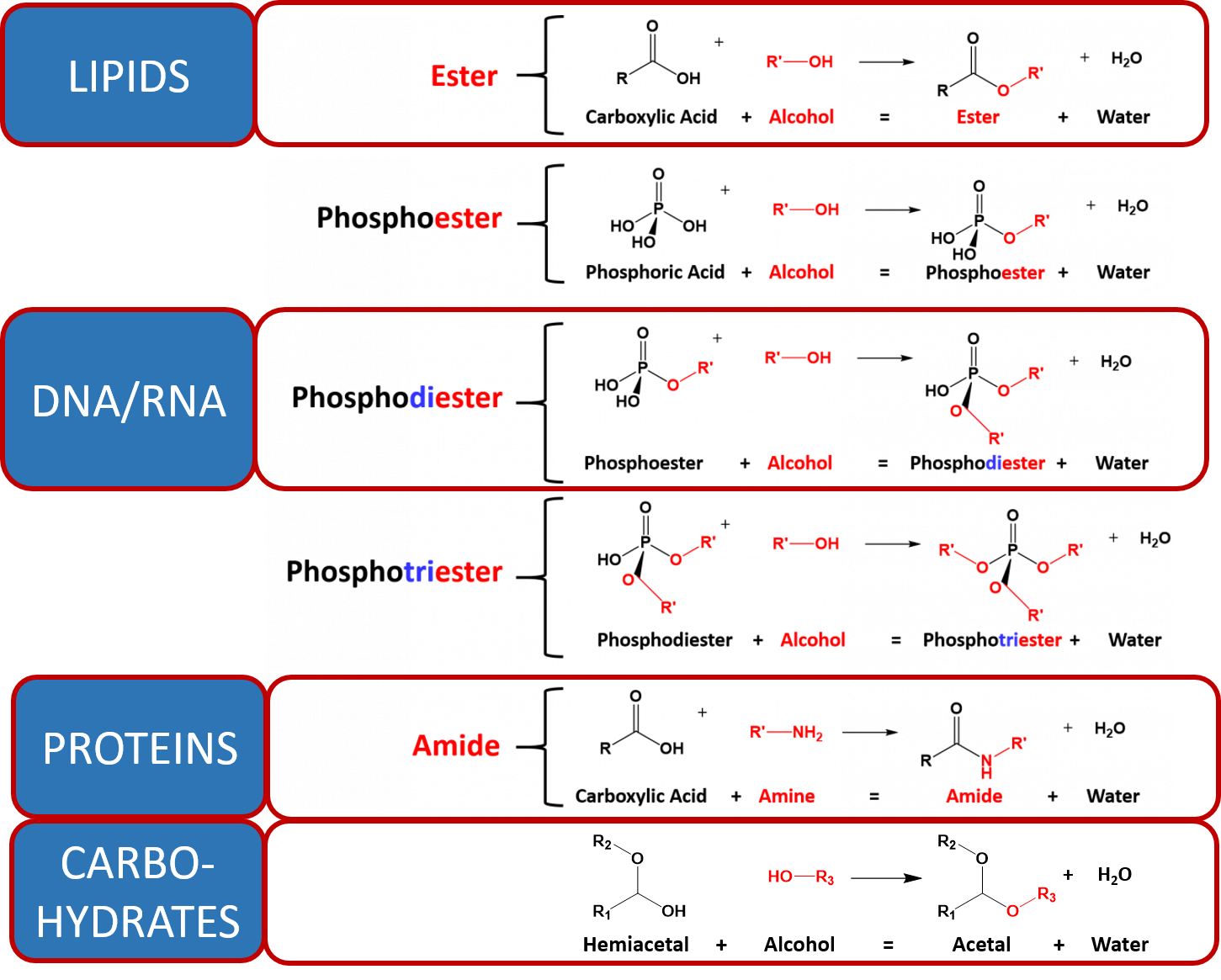
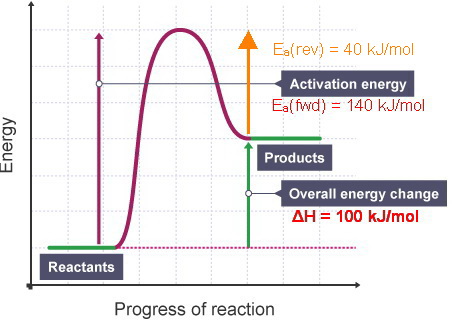
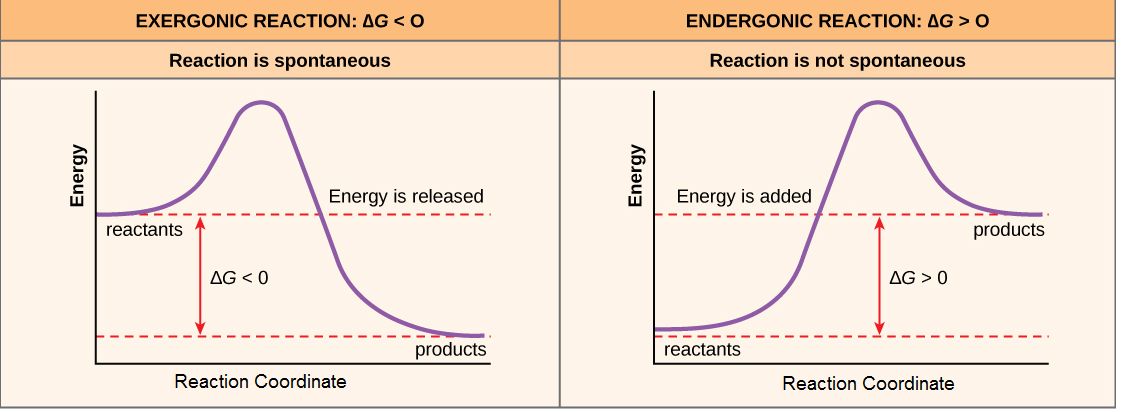


/endergonic-vs-exergonic-609258_final-2904b2c359574dfcb65a9fca2d54179a.png)

















0 Response to "39 the diagram below represents a spontaneous reaction"
Post a Comment