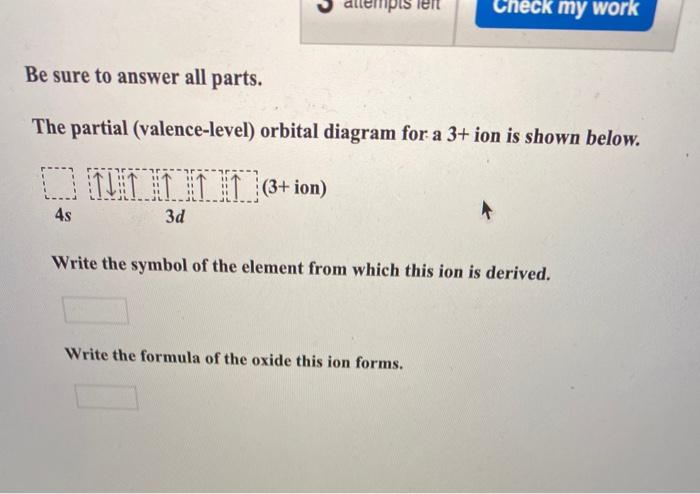
38 the partial (valence-level) orbital diagram for a 3+ ion is shown below.
Orbital Diagram For Aluminum.They consist of the symbol for the element in the. It explains how to write the orbital diagram.Electron Dot Diagram For Aluminum — UNTPIKAPPS (Millie Bailey) Here are some orbital diagram s of elements with more electrons to help you understand the rules, electron configuration, orbital diagram s, and quantum numbers. . They consist of the symbol for the element ...
To obtain the molecular orbital energy-level diagram for \(\ce{O2}\), we need to place 12 valence electrons (6 from each O atom) in the energy-level diagram shown in Figure 9.10.1 . We again fill the orbital s according to Hund's rules and the Pauli principle, beginning with the orbital that is lowest in energy.
3.35. A cation is a positively charged ion with one or fewer electrons than its neutral atom. An anion is a negatively charged ion with one or more electrons than its neutral atom. A polyatomic ion is made up of more than one atom; the whole unit is the ion. 3.36. Titanium lost four electrons to form Ti4+; it has 22 protons and 18 electrons. 3 ...
The partial (valence-level) orbital diagram for a 3+ ion is shown below.
The Mo L 3-edge spectra for Mo 5 N 6 (Fig. 6a) show that the valence state of Mo decreases gradually with the discharge and reaches lowest level at the discharge potential of 0.5 V.
1. Determine the total number of valence electrons in the molecule or ion. For CO32−, for example, we add two electrons to the total because of the −2 charge. 2. Arrange the atoms to show specific connections. 3. Place a bonding pair of electrons between each pair of adjacent atoms to give a single bond. 4.
Figure 9.7. 3: Molecular Orbital Energy-Level Diagrams for Diatomic Molecules with Only 1 s Atomic Orbitals. (a) The H 2+ ion, (b) the He 2+ ion, and (c) the He 2 molecule are shown here. Figure 9.7. 3 a shows the energy-level diagram for the H 2+ ion, which contains two protons and only one electron.
The partial (valence-level) orbital diagram for a 3+ ion is shown below..
Honda Illustrated Parts Diagram s : Honda GXV160 Engine Part Diagram: No: Description: Original Honda Part: Aftermarket Honda Replacement Part: 1 Starter Assembly OEM Honda Part No: 28400-ZG9-802: This part is no longer available and has been replaced by Honda 28400-ZG9-803. Aftermarket Part No: 31-070: 1-16 of over 1,000 results for "honda gcv160 parts" Price and other details may vary based ...
Steps to form OF2 Lewis Structure Diagram. Step 1: Find the Total number of Valence Electrons. The first and foremost step is to calculate the total number of valence electrons in an OF2 molecule. Oxygen belongs to group 16, the chalcogen family, and has a valency of 6. Fluorine belongs to the family of halogen in group 17 and has a valency of 7.
Record up to 21 points here. Score up to 3 points for each, so that you can award partial credit. List the five reaction types and give a standard formula using variables (A, B, etc.) or an example if you can’t remember the other. 5 points for knowing the names and 5 points for the example or formula for each. Score up to 10 total points here.
Lesson 2 Venn Diagram in pairs.doc Week 16 Smartboard Notes.pdf Week 17 H and outs / Power Points Ellis Isl and and Angel Isl and Constructed Response.doc Lesson 6 Limits on Immigration.doc Limits on Immigration Nativists Exclusion Act.ppt Immigration Acts Notes.doc Immigration Review Sheet.doc ImmigrationTest Review Notes.doc Week 17 Smart Board ... data:image/png;base64 ...
The electron configuration of aluminium chemical element is Ne3s23p1 Ne 3 s 2 3 p 1 or 1s22s22p63s23p1 1 s 2 2 s 2 2 p 6 3 s 2 3 p 1. So although a neutral atom of aluminum has 13 electrons the ion of aluminum Al 3 has lost three electrons and only has 10.
The electron configurat ion of a neutral magnesium atom is: 1s2 2s2 2p6 3s2 or in shorthand [Ne] 3s2. ... For the cantilever beam shown below, draw the free body diagram and determine the reactions at the fixed s... 40 the partial (valence-level) orbital diagram for a 3+ ion is shown below.
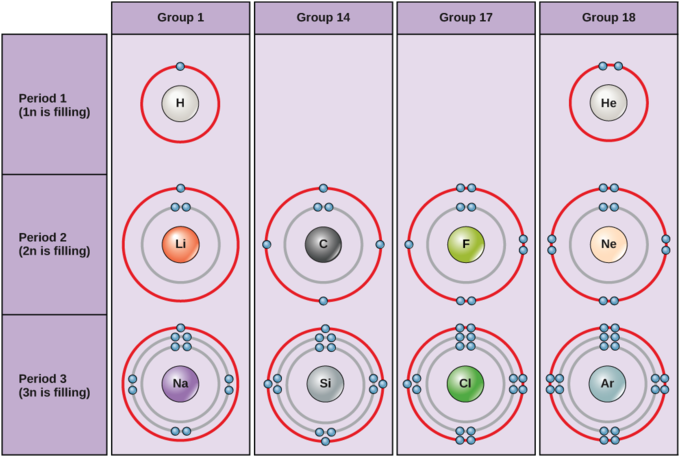
The general valence shell electronic configuration of p-block elements is ns 2 np 1-6 (except for He).The inner core of the electronic configuration may,however,differ.The General electronic configuration shown by elements from group13 to 18 of p-block is as given below : Group 13 (Boron family) - ns 2 np 1. Group 14 (Carbon family) - ns 2 np 2
Chemistry Archive: Questions from December 13, 2021. HW Iron-Iron Carbide Diagram Use Your own Diagram to solve the following problems . A. A steel is cooling slowly from liquid condition until to room temperature. When it passes through temperature per. 0 answers.
Figure 3.8.1: This is if nitrogen monoxide has only ten valence electrons, which it does not. Let's look at the formal charges of Figure 3.8.2 based on this Lewis structure. Nitrogen normally has five valence electrons. In Figure 3.8.1, it has two lone pair electrons and it participates in two bonds (a double bond) with oxygen.
Undergrad. (yrs 3-4) Logistics. 2. View this sample Research paper. Reflecting on Adult Education/Training. Master's. Education. 6. View this sample Essay (any type) Cultural Activity. Undergrad. (yrs 3-4) History. 2. View this sample Creative writing. Creating a Culture of Innovation. Undergrad. (yrs 1-2) Management. 4 ...
Create a plot diagram to identify the plot elements and at least 3 major techniques used in the text you have been reading independently. Each oxygen also has 2 non-bonding pairs of electrons. Base your answers to questions 22 and 23 on the information below.
A partial motion diagram is shown in figure above. ... For the cantilever beam shown below, draw the free body diagram and determine the reactions at the fixed s... 40 the partial (valence-level) orbital diagram for a 3+ ion is shown below. Electron configurat ion s of transit ion metal elements Hydrogen Z 1. Lithium Z 3. Ce 3 Xe4f 1.
Therefore, the 3s-orbital has (3 - 1) = 2 radial nodes, as shown in the. We find that when the ratio of the ionic thermal de Broglie wavelength to the mean interionic distance is larger than about 0. Figure 2: Radial distribution function for a dense liquid. Ask Question. 4 Electron Configurations and Valence Electrons.
The less stabilizing orbital interaction between (H 2 O) 3 ···F - and C 2 H 5 Cl can be ascribed to the difference in the orbital energies of their interacting lone pair HOMOs. As shown in Figure 2c, the HOMO of (H 2 O) 3 ···F - is lower (i.e., more stable) than that of bare F -.
The bond order is Figure The molecular orbital energy-level diagram for both the NO+ and CN-ions. Figure A partial molecular orbital energy-level diagram for the HF molecule. This interaction introduces an element of s-p mixing, or hybridization, into the molecular orbital theory. The. Molecular geometry of Cl2 Hybridization of Cl2.
Lewis dot structures are one way to represent how atoms form covalent bonds. A table of Lewis dot symbols of nonmetal elements that form covalent bonds is shown in Fig. 2.28 Dots are placed around the symbol of the element to represent the number of valence electrons in the element. There can be up to eight dots, for eight valence electrons.
This might seem to correspond to Na 6 Cl 6, but note that the central sodium ion shown in the diagram can claim only a one-sixth share of each of its chloride ion neighbors. Therefore, the formula NaCl is not just the simplest formula, but correctly reflects the 1:1 stoichiometry of the compound.
MAPbBr 3 precursor solution was prepared by dissolving MABr and PbBr 2 with the molar ratio of 1:1 in DMF. Then, MAPbBr 3 films were prepared in a nitrogen-filled glovebox by spin-coating the precursor solution (with the concentration of 0.6 M) onto ITO at a speed of 6000 rpm. During this process, a drop of chlorobenzene was added to obtain a ...
On the one hand, the highest occupied molecular orbital (HOMO) for PEDOT:PSS is only about 5.1 eV, compared to the valence band of the perovskite layer (5.4 eV) , which leads to important losses in the open-circuit voltage (V oc) of the devices, causing a V oc below 1 V [10,11].
The two resonances have different partial-wave contributions as shown in fig. S9, with the main contributions for symmetry a 1 being l = 0, 3, and 4, while for symmetry t 2, it is l = 1, 2, and 3. Qualitatively, the asymmetry in the RAPID of SB12 can be described by a single-channel calculation for a 1 symmetry using only the dominant partial ...
The orbital diagram of formic acid, which represents the sigma bonds, is shown below. We can observe from the orbital diagram is that the carbon atom is the sp2 hybridized and one of the oxygen atoms is also sp2 hybridized whereas another oxygen atom bonded to hydrogen and carbon atom, is sp3 hybridized.
Electronic configuration Valency Ion formed Fluorine. 28 3 Al3 An Aluminium atom needs to lose 3 electrons to attain a noble gas electronic structure. Nearest Noble gas. An electron shell is the set of allowed states that share the same principal quantum number n the number before the letter in the orbital label that electrons may.
16.11.2018 16.11.2018 4 Comments on 2013 Open Range 413ll Trailer Wiring Diagram. ...Jul 16, · Highland RV Open Range RLL Nielson RV Open Range RLS Used Triple Slide Wide Body & Deep Tour Our Grand Design Imagine MK Travel Trailer, Our Rig For Full Time. Recent Open Range Open Range RLL questions, problems & answers. DC is for Priorities - AC is for the Extras. The most obse rv ant among you ...
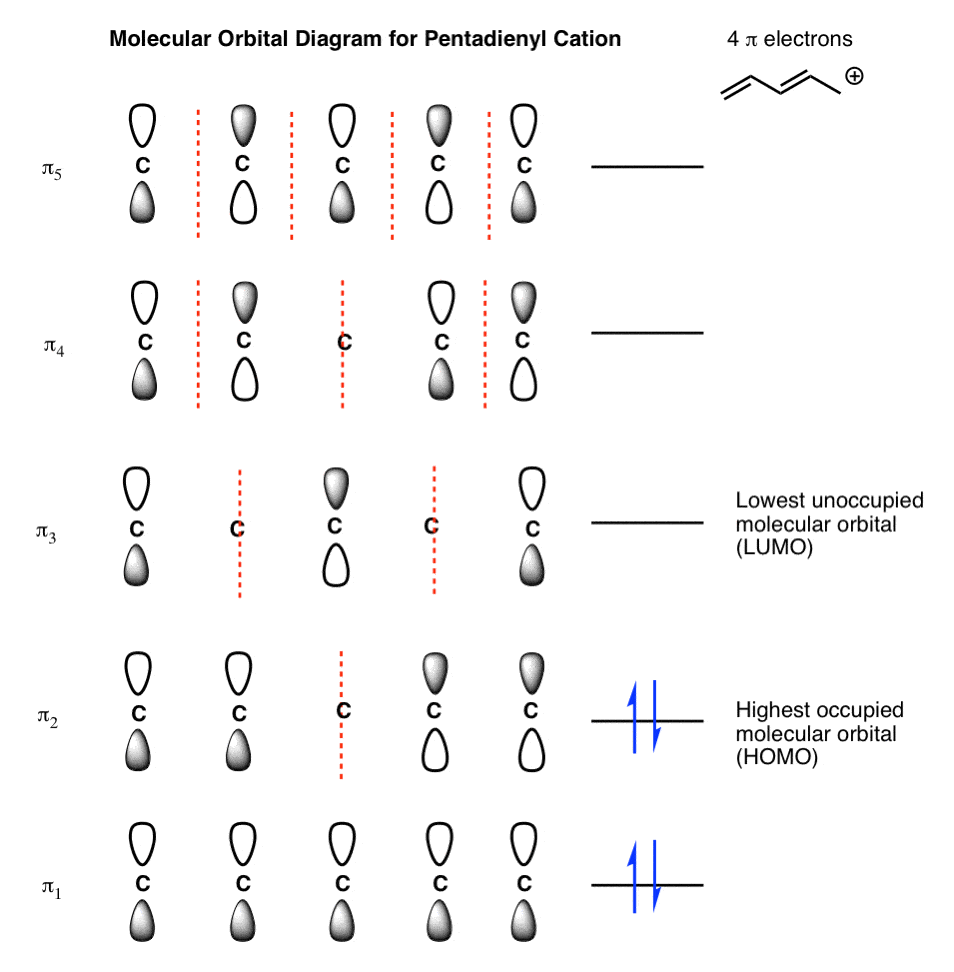
Benzene has 3 double bonds, so it has 6 π electrons. Its first 2 π electrons fill the lowest energy orbital, and 4 π electrons remaining fill in the orbitals of the succeeding energy level. Notice how all of its bonding orbitals are filled, but none of the anti-bonding orbitals have any electrons. To apply the 4n+2 rule, first count the ...
8.5 In the Lewis structure shown here, A, D, E, Q, X, and Z represent elements in the first two rows of the periodic table (H− Ne). Identify all six elements so that the formal charges of all atoms are zero. [Section 8.3] 8.6 Incomplete Lewis structures for the nitrous acid molecule, HNO 2, and the nitrite ion, , are shown below.
Molecular orbital diagram for the molecule oxygen o2. Molecular orbital diagram for f2. A fundamental principle of these theories is that as atoms bond to form molecules a certain number of atomic orbitals combine to form the same number of. C would this ion exist. To further demonstrate the consistency of the lewis structures with mo. For the ...
Electron Configurations and Orbital Diagrams Electron configuration is indicated by a shorthand notation: # of electrons in the sublevel Orbital diagrams make use of a box, circle, or line for each orbital in the energy level. The diagram on the left shows the orbitals in order of increasing energy.
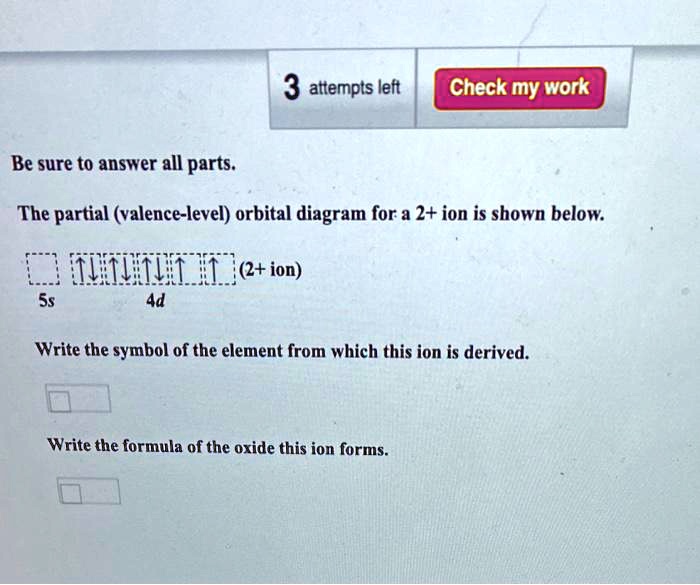

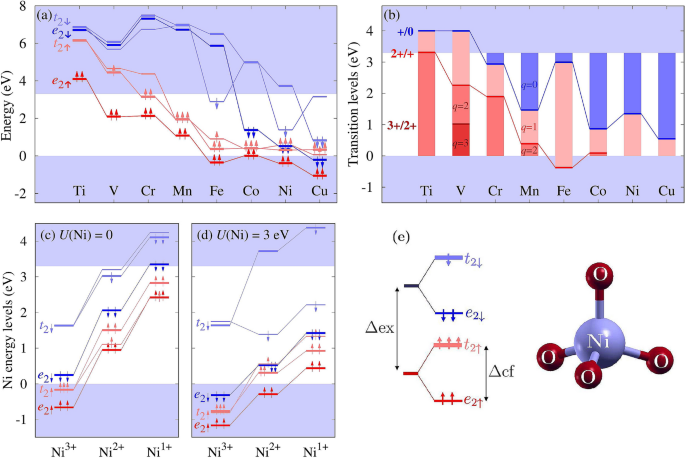
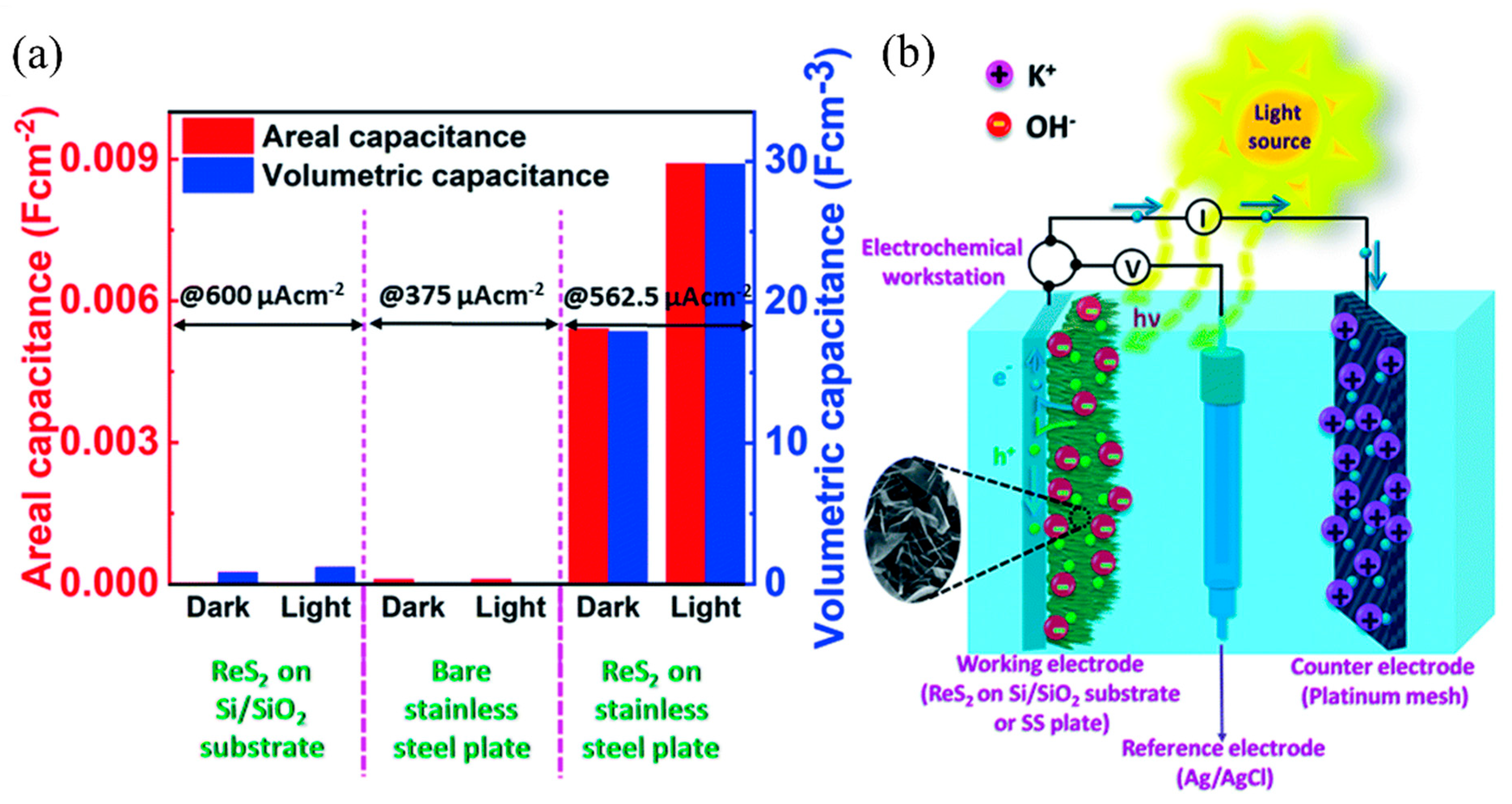
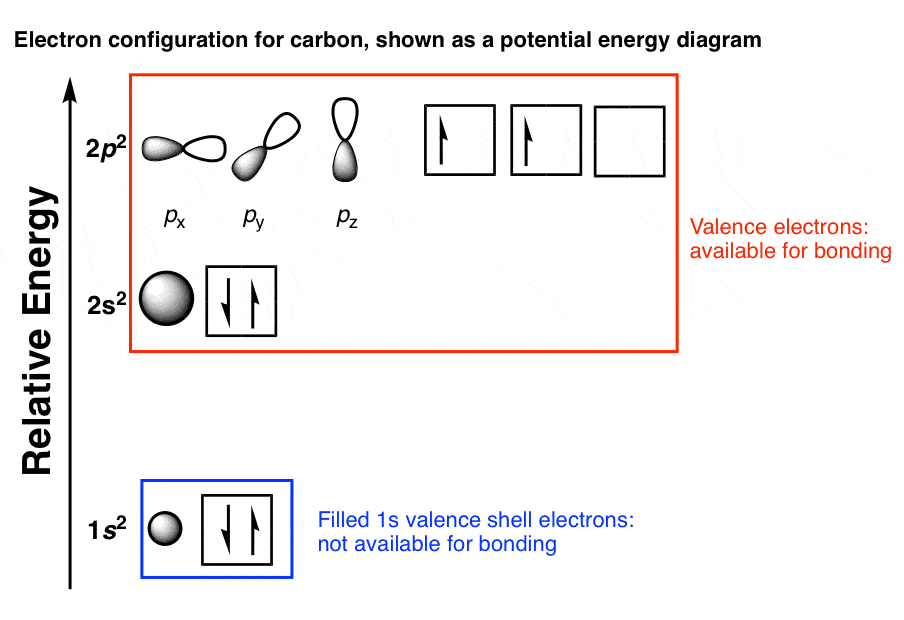




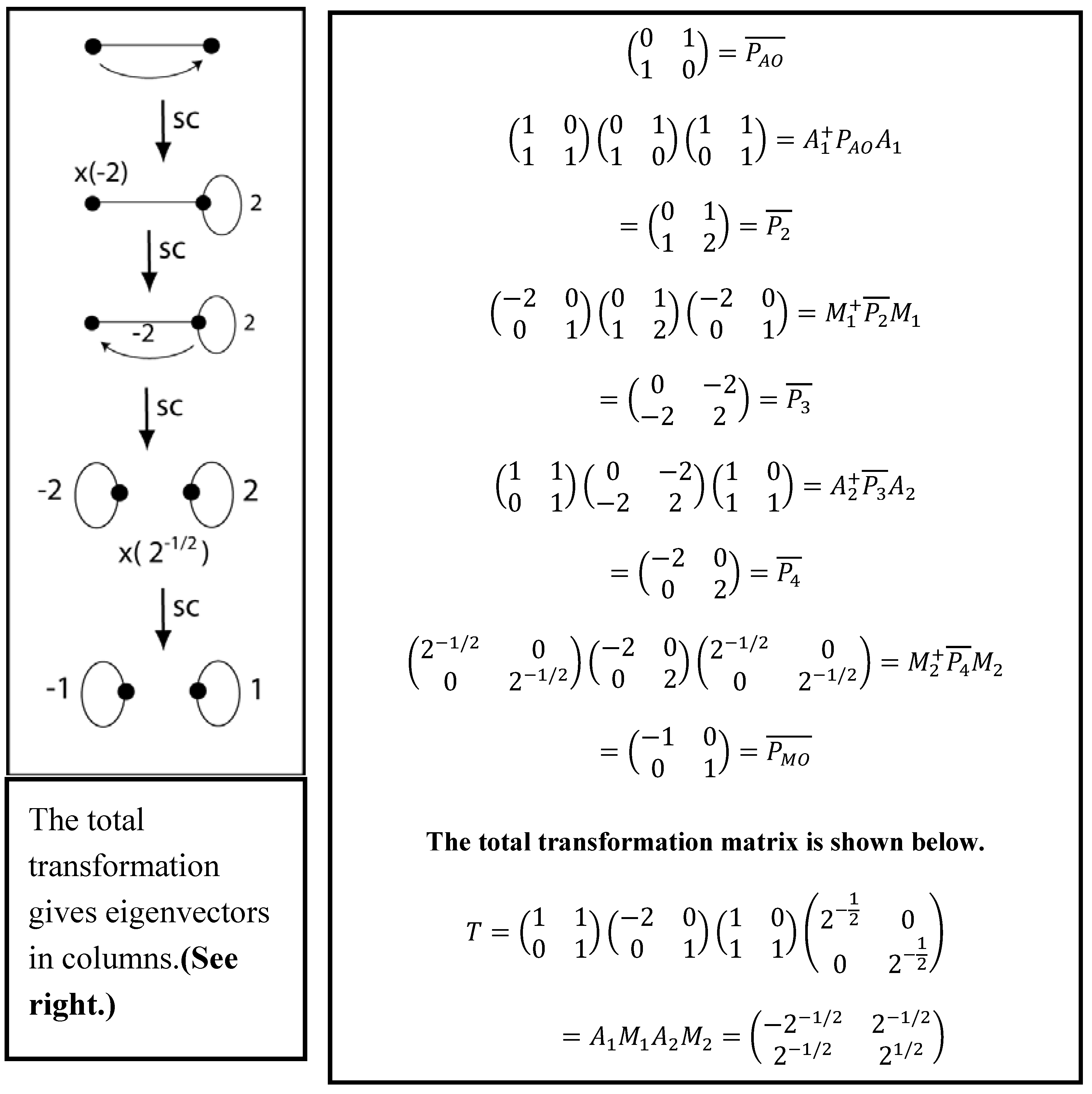

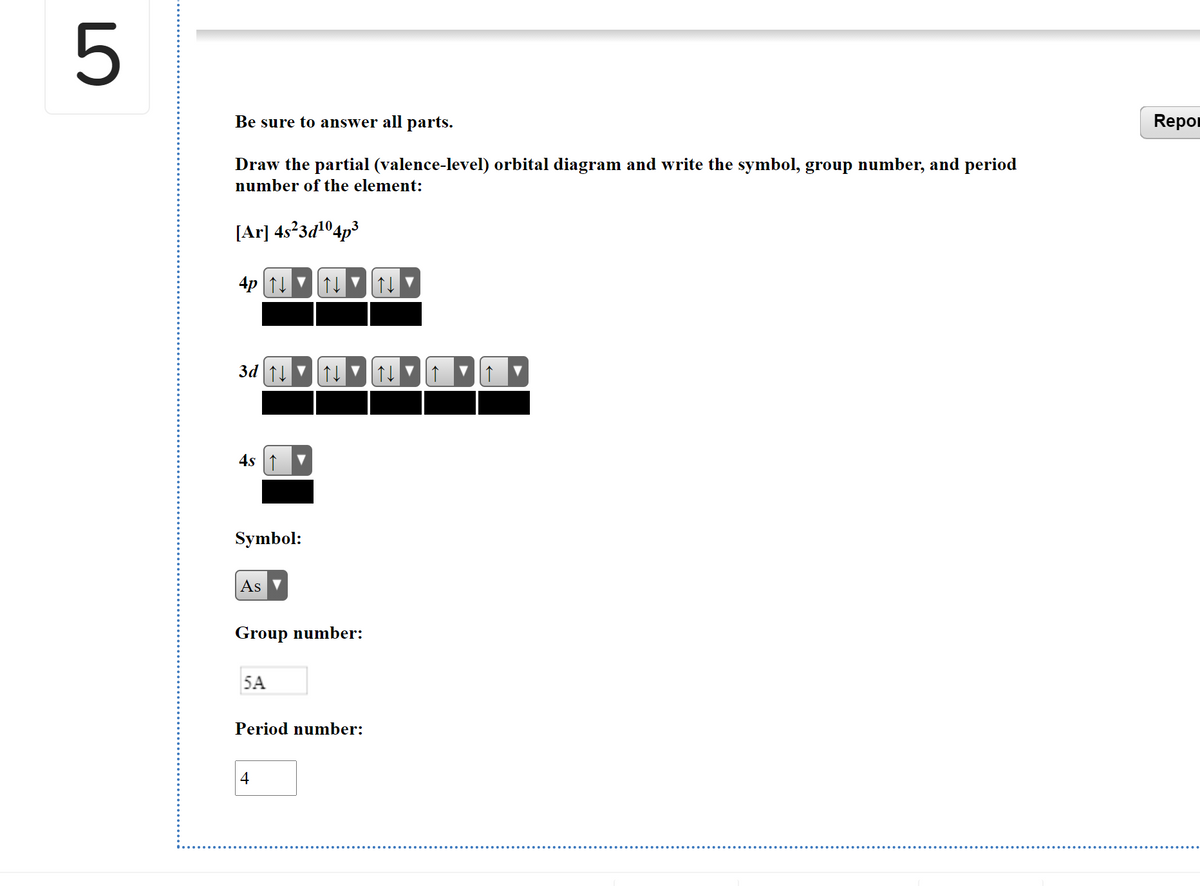

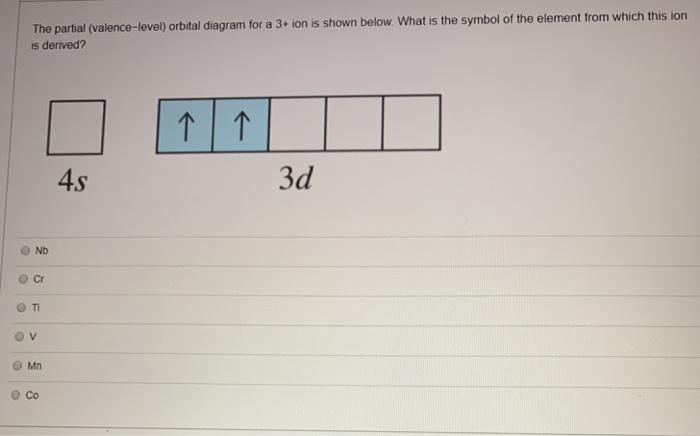



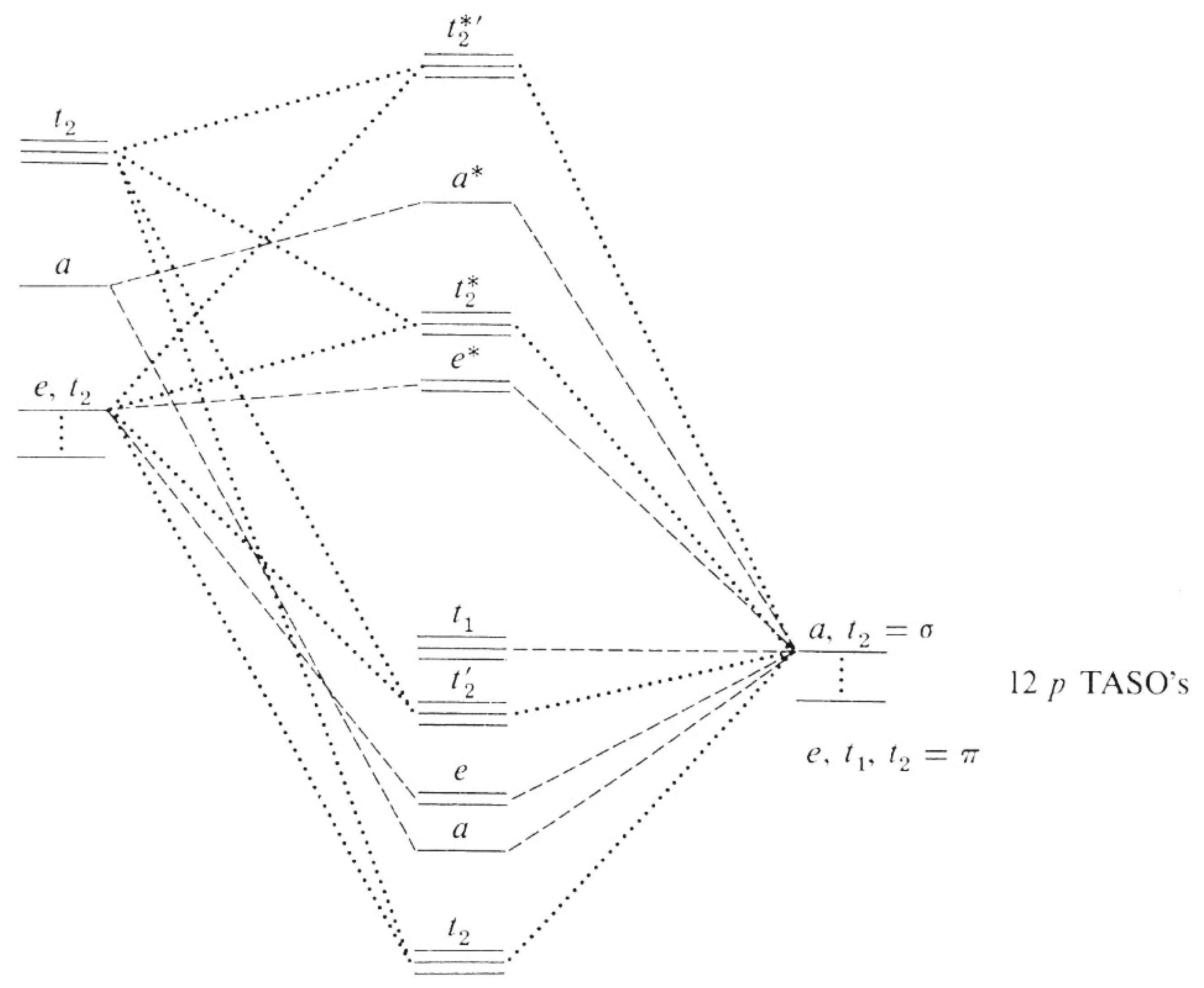



0 Response to "38 the partial (valence-level) orbital diagram for a 3+ ion is shown below."
Post a Comment