42 the line connecting the triple point and the critical point on a phase diagram represents _____.
This photo about: The Line Connecting the Triple Point and the Critical Point On A Phase Diagram Represents _____., entitled as Phase Diagrams The Line Connecting The Triple Point And The Critical Point On A Phase Diagram Represents . - also describes Phase Diagrams and labeled as: mirror online,storyline online,the line app,the line haircut,the line train, with resolution 2986px x 1133px Academia.edu is a platform for academics to share research papers.
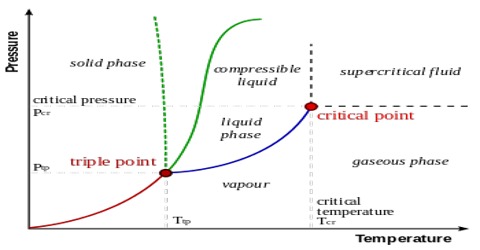
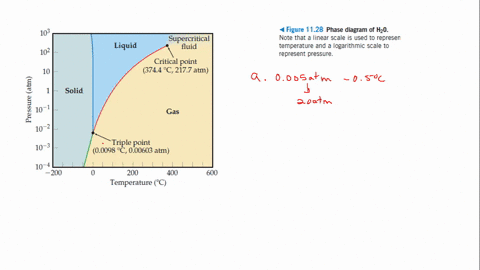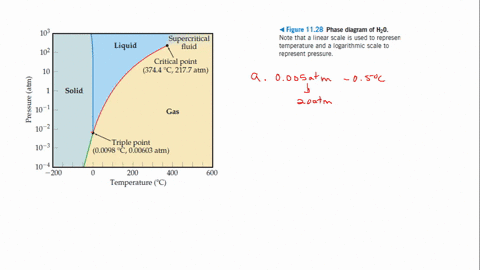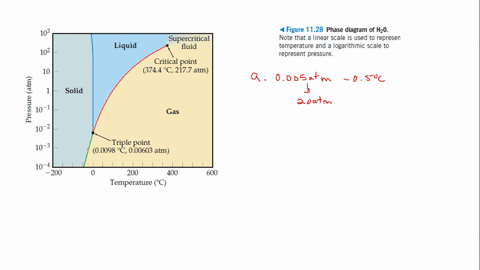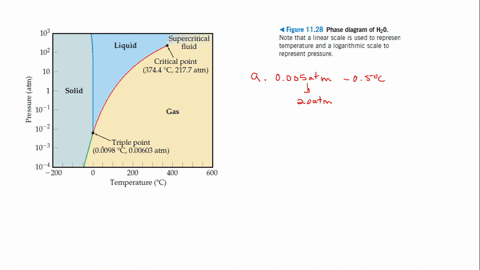
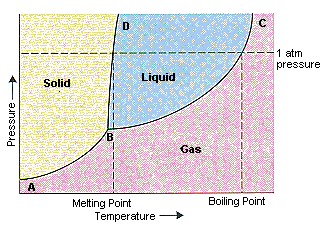
Every point in this diagram represents a possible combination of temperature and pressure for the system. The diagram is divided into three areas, which represent the solid, liquid, and gaseous states of the substance. The best way to remember which area corresponds to each of these states is to remember the conditions of temperature and pressure that are most likely to be associated with a ...

The line connecting the triple point and the critical point on a phase diagram represents _____.
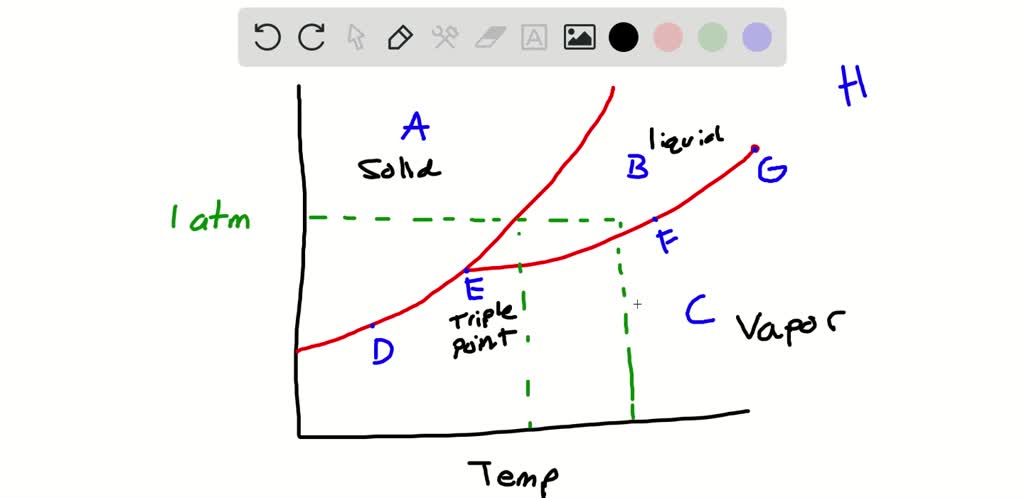
A) The triple point of this substance occurs at a temperature of 31°C. B) At 10 atm of pressure, there is no temperature where the liquid phase of this substance would exist. C) The solid phase of this substance is higher in density than the liquid phase. D) The line separating the solid and liquid phases represents the ΔH vap. 20.04.2015 · When a soldering iron is switched on, the iron takes time to reach the solder’s melting point. Simply connect this circuit to the soldering iron as shown in the figure and the iron reaches the ... Transcribed image text: In the phase diagram shown above, the coordinates of point _____ correspond to the critical temperature and pressure. A B C D E The effect of ...
The line connecting the triple point and the critical point on a phase diagram represents _____.. Cite. Deleted profile. There is difference between them ,the triple point or called the single phase point represent the state in which the solid,liquid and vapor exist together, when the critical ... Triple point. We can see triple point in above phase diagram of water. Phase diagram of water will locate one point in curve at which all phases of water will coexist together. At triple point, pressure will be 4.58 mm of Hg and respective temperature will be 0.00750C. Let us see one live example to understand the concept of triple point or ... The line connecting the triple point and the critical point on a phase diagram represents _____. a) the temperatures and pressures above which only a supercritical fluid can exist. b) the temperatures and pressures at which the solid and gas states are equally stable and at equilibrium. c) the temperatures and pressures at which the liquid and ... Step 6: Critical path diagram to show project progresses. Critical path diagram is a live artefact. Therefore, this diagram should be updated with actual values once the task is completed. This gives more realistic figure for the deadline and the project management can know whether they are on track regarding the deliverables.
The triple point is the point on the phase diagram where the lines of equilibrium ... of most substances, the solid lines represent the phase boundaries. Academia.edu is a platform for academics to share research papers. For example, the water phase diagram has a triple point corresponding to the single temperature and pressure at which solid, liquid, and gaseous water can ... Identify phase equilibrium lines, triple points and critical points on a phase diagram Describe at what point a substance is a supercritical fluid To unlock this lesson you must be a Study.com Member.
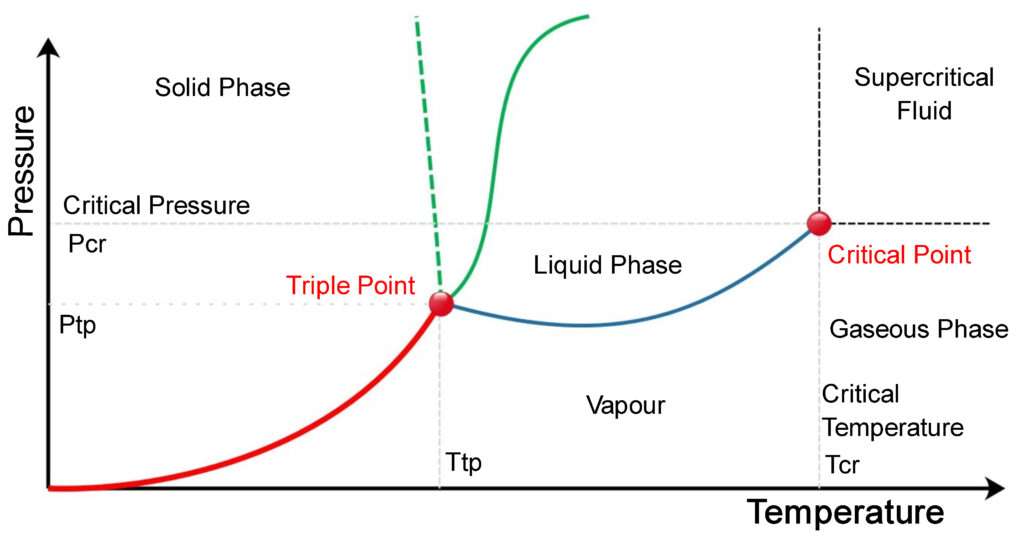
Project managers have long relied on work breakdown structures (WBS) to help them effectively and efficiently plan and implement projects. As the discipline has matured, project professionals have expanded and matured the WBS concept. This paper examines the dimensions that are involved in developing a WBS that can help project teams successfully realize their project goals. The point at which the lines intersect represents the triple point. At the pressure and temperature of the triple point, all three phases (solid, ... On the contrary, there is almost no voltage fluctuation in the three-phase neutral-point-clamped inverter and three-phase full-bridge inverter with split capacitor structure. Since there is a small voltage fluctuation, the earth leakage current to be formed is more advantageous as it complies with the standards. If three-phase full-bridge inverter topology is used, lower fluctuation occurs in ... There are also two important points on the diagram, the triple point and the critical point. The triple point represents the combination of pressure and temperature that facilitates all phases of matter at equilibrium. The critical point terminates the liquid/gas phase line and relates to the critical pressure, the pressure above which a ...
This chemistry video tutorial explains the concepts behind the phase diagram of CO2 / Carbon Dioxide and the phase diagram of water / H2O. This video contai...
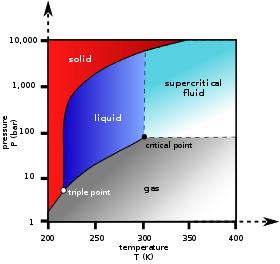
12.4: Phase Diagrams. To understand the basics of a one-component phase diagram as a function of temperature and pressure in a closed system. To be able to identify the triple point, the critical point, and four regions: solid, liquid, gas, and a supercritical fluid. The state exhibited by a given sample of matter depends on the identity ...
The Ontario Curriculum, Grades 1-8 | The Arts The Arts, 2009 (revised) INTRODUCTION This document replaces The Ontario Curriculum, Grades 1 8: The Arts, 1998. Beginning in Septe
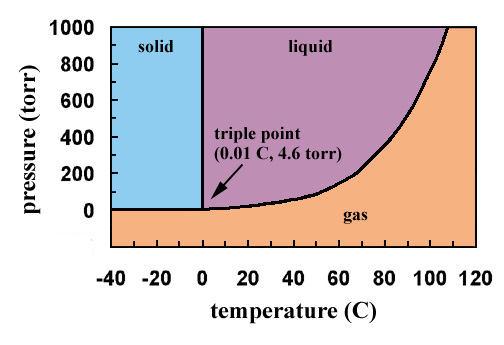
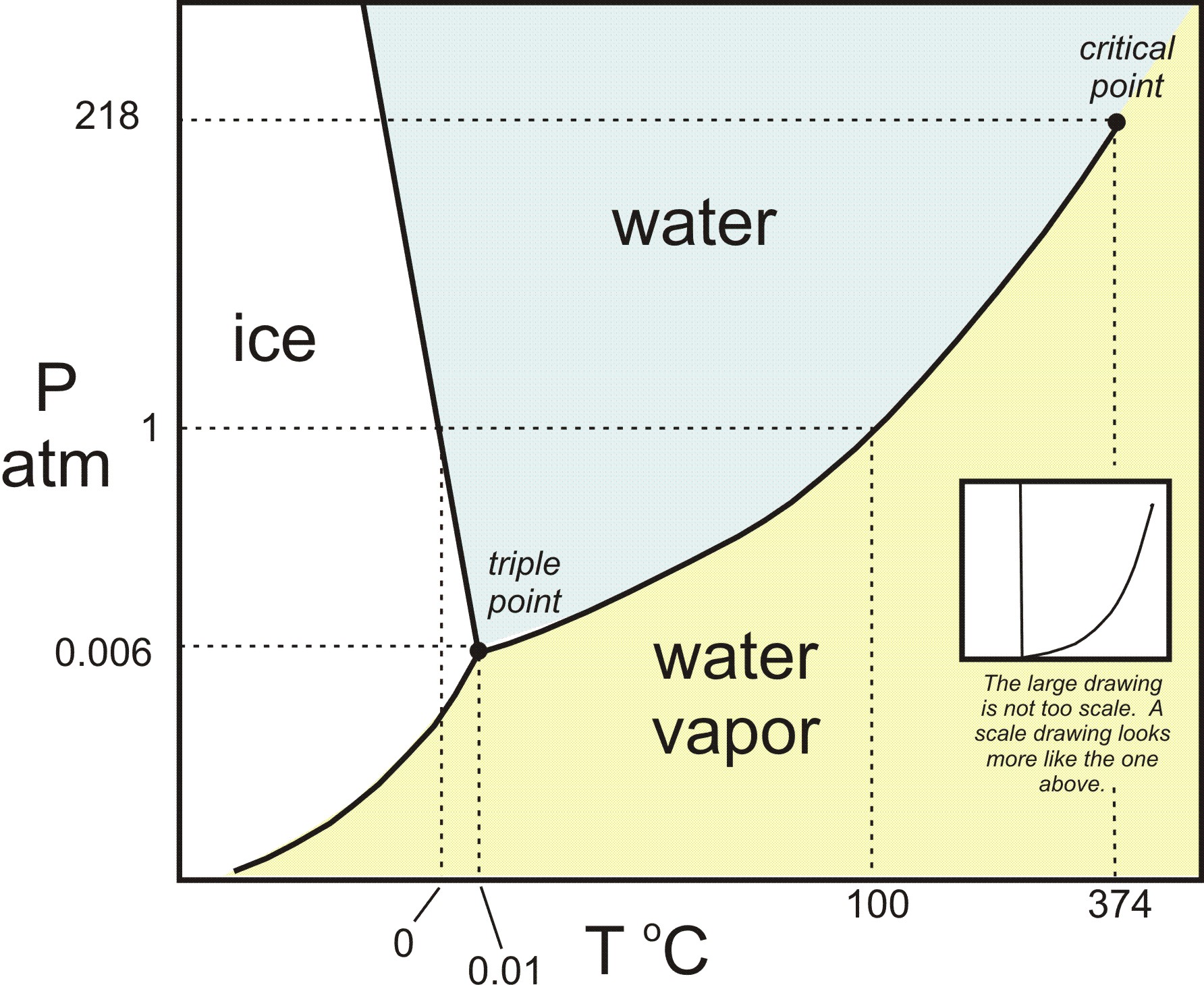
Triple Point: The point O where all the three curves OC, OA and OB meet is known as triple At the triple point all the three phases of water system namely solid ice, liquid water and gas vapour are in equilibrium. The equilibrium in three phases is attained at 0.0076 0 C temperature and 4.58 mm Hg pressure. The degree of freedom will be : F = C - P + 2 = 1 - 3 + 2 = 0
The triple point is merely the point of intersection of the sublimation and vaporization curves, It has been found that on a 'p-T' diagram the triple point is represented by a point (Fig. 19.6) and on a 'p-v' diagram it is a line (Fig. 19.3), and on a 'u-v' diagram it is a triangle.
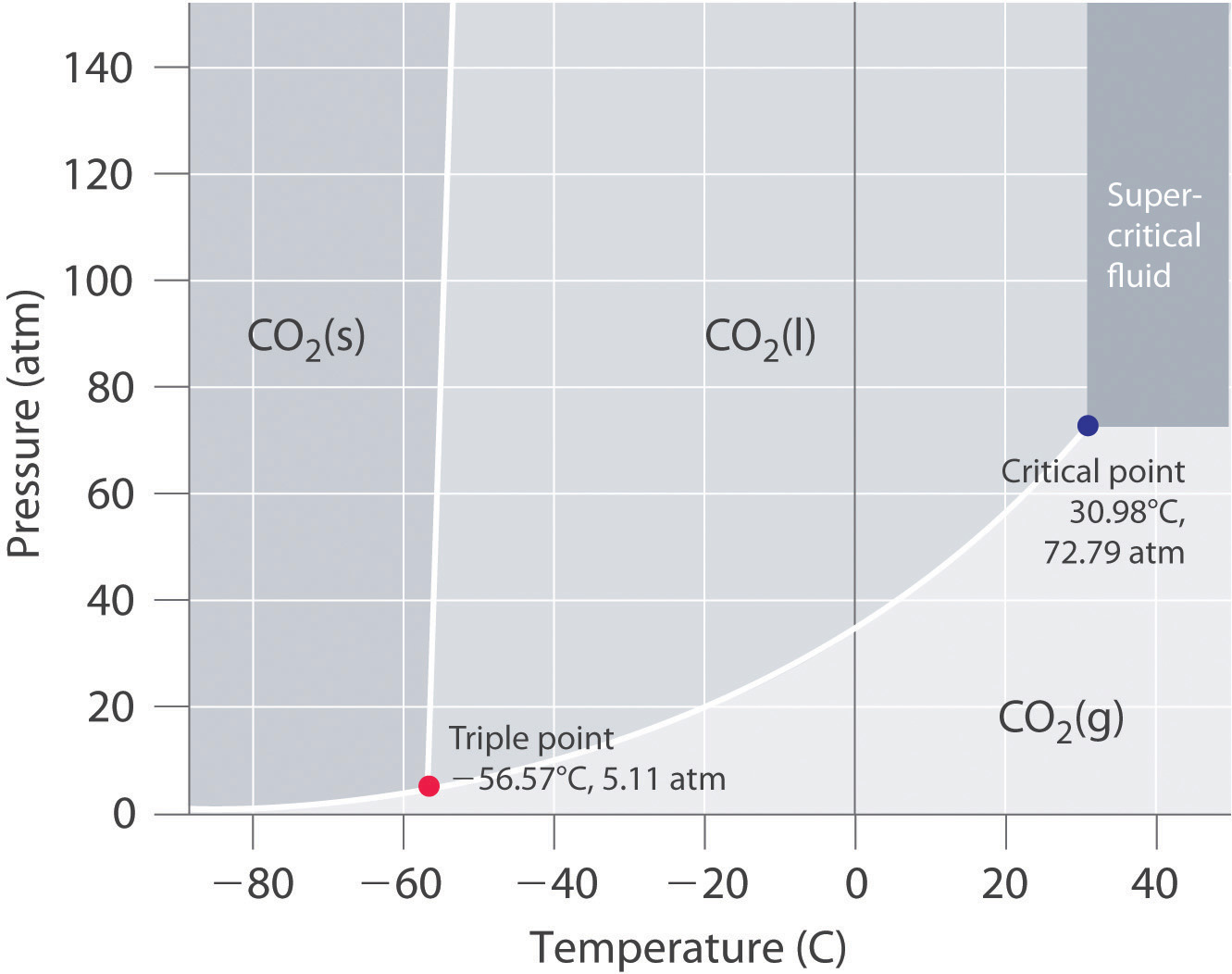
Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating that carbon dioxide cannot exist as a liquid ...
The line connecting the triple point and the critical point on a phase diagram represents _____. the temperature and pressure combinations above which only a supercritical fluid can exist the temperature and pressure combinations at which the liquid and solid states are equally stable and at equilibrium
The triple point on the diagram is where a substance is a solid, liquid, and a gas all at the same time; the three states are in equilibrium and any change will cause all of the material to be in ...
Figure 3.6: P-v Diagram And Phase Envelope Of A Pure Substance. If you carefully follow the trend of the critical isotherm (@ T = T c in Fig. 3.5), you will realize that it has a point of inflexion (change of curvature) at the critical point. Furthermore, the critical point also represents the maximum point (apex) of the P-v envelope.
KEY: Phase Diagram Worksheet (1) (2) Normal Melting Point = -7.0°C Normal Boiling Point = 58.5°C Triple Point = -8°C and 6 kPa (3) See answer to 1. (4) The melting point curve leans slightly to the right (has a positive slope) indicating that, as pressure is increase, the melting point of bromine increases.
Chapter 9 - 10 Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram
The line connecting the triple point and the critical point on a phase diagram represents. a) the temperatures and pressures above which only a supercritical fluid can exist. b) the temperatures and pressures at which the solid and gas states are equally stable and at equilibrium. c) the temperatures and pressures at which the liquid and solid ...
At pressures below the triple point, a substance cannot exist in the liquid state, regardless of its temperature. The terminus of the liquid-gas curve represents the substance's critical point, the pressure and temperature above which a liquid phase cannot exist.
The line connecting the triple point and the critical point on a phase diagram represents. The triple point of a phase diagram is the location where the solid liquid and gas phases meet. At triple point all three phases solid liquid and gas are in equilibrium means these cant be separated at triple point.
To be able to identify the triple point, the critical point, ... A typical phase diagram consists of discrete regions that represent the ...
The Line Connecting The Triple Point And The Critical Point On A Phase Diagram Represents _____. the temperature and pressure combinations above which only ...
The triple point. Point T on the diagram is called the triple point. ... These can be found from the phase diagram by drawing a line across at 1 atmosphere pressure. ... Now lets put some numbers on the diagram to show the exact positions of the critical point and triple point for water.
Lines of excitation frequencies are then drawn vertically on the Campbell diagram, and a line corresponding to the design speed is drawn horizontally. Where the lines of excitation frequencies and multiples of running speed intersect near the line of design rpm, a problem area may exist. If, for instance, an impeller has 20 blades, a design speed of 3,000 rpm (50 Hz), and a critical frequency ...
The Line Connecting the Triple Point and the Critical Point On A Phase Diagram Represents _____. solved the line connecting the triple point and the criti answer to the line connecting the triple point and the critical point on a phase diagram represents a the temperatures and what do the triple point and critical point on a phase the triple point of a phase diagram is the the critical point ...
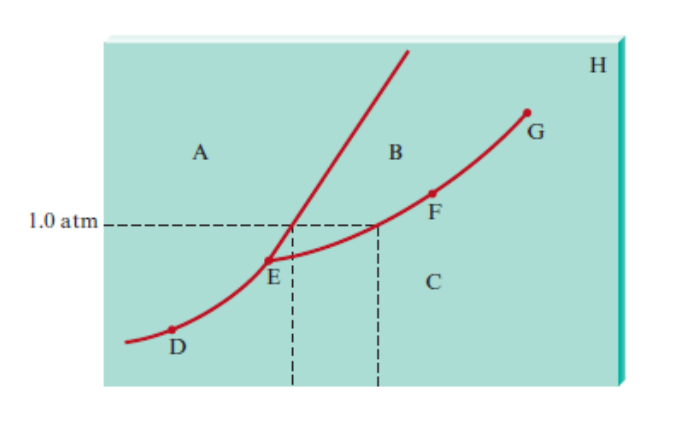
phase diagram. A point on any of these lines can represent any two-phase mixture at that particular temperature and pressure. The triple line of the p-v-T surface projects onto a point on the phase diagram called the triple point. Three phases coexist on the triple line or the triple point. 2 M or an , .J dS h piH N Fu m et lsf E g T yc W 2 0 83
3. The degree of freedom at triple point in unary diagram for water _____. (a) 0 (b) 1 (c) 2 (d) 3 4. Above the following line, liquid phase exist for all compositions in a phase diagram. (a) Tie-line (b) Solvus (c) Solidus (d) Liquidus 5. Following is wrong about a phase diagram. (a) It gives information on transformation rates.
a) The point at which the solid, liquid and gaseous phases for a substance co-exist. b) The triple point exists for a substance occurs at a specific temperature and pressure. c) The triple point exists at a single temperature and is independent of pressure. d) The system must be enclosed so that no vapour can escape.
The line connecting the triple point and the critical point on a phase diagram represents _____. the temperature and pressure combinations above which only a supercritical fluid can exist the temperature and pressure combinations at which the liquid and solid states are equally stable and at equilibrium the temperature and pressure combinations at which the liquid and gas states are equally ...
the critical point is the temperature and pressure beyond which the gas and liquid phases are indistinguishable. What is the significance of the triple point on a phase diagram? the triple point on a phase diagram represents the temperature at which the gas, liquid and solid phases are in equilibrium ...
It is therefore called the triple point of the substance, and it represents the only point in the phase diagram in which all three states are in equilibrium. Point C is the critical point of the substance, which is the highest temperature and pressure at which a gas and a liquid can coexist at equilibrium.
Transcribed image text: In the phase diagram shown above, the coordinates of point _____ correspond to the critical temperature and pressure. A B C D E The effect of ...
20.04.2015 · When a soldering iron is switched on, the iron takes time to reach the solder’s melting point. Simply connect this circuit to the soldering iron as shown in the figure and the iron reaches the ...
A) The triple point of this substance occurs at a temperature of 31°C. B) At 10 atm of pressure, there is no temperature where the liquid phase of this substance would exist. C) The solid phase of this substance is higher in density than the liquid phase. D) The line separating the solid and liquid phases represents the ΔH vap.















0 Response to "42 the line connecting the triple point and the critical point on a phase diagram represents _____."
Post a Comment