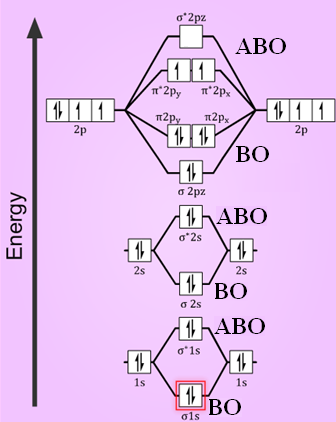
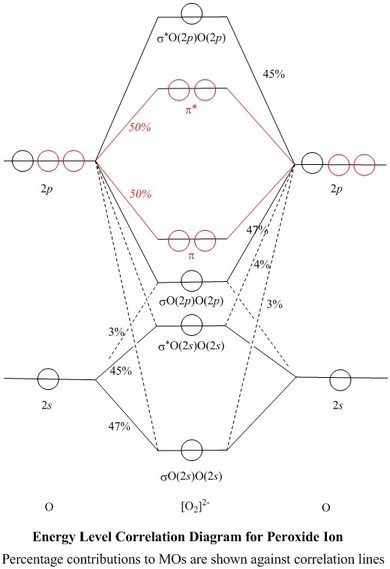
40 molecular orbital diagram for o2- ion
1610s, "an illustrative figure giving only the outlines or general scheme of the object;" 1640s in geometry, "a drawing for the purpose of demonstrating the properties of a figure;" from French diagramme, from Latin diagramma "a scale, a musical scale," from Greek diagramma "geometric figure, that which is marked out by lines," from diagraphein "mark out by lines, delineate," from dia "across, through" (see dia-) + graphein "write, mark, draw" (see -graphy). Related: Diagrammatic; diagrammatically. The verb, "to draw or put in the form of a diagram," is by 1822, from the noun. Related: Diagrammed; diagramming. However, one of the most important molecules we know, the oxygen molecule O2, ... Obtain the molecular orbital diagram for a homonuclear diatomic ion by ...
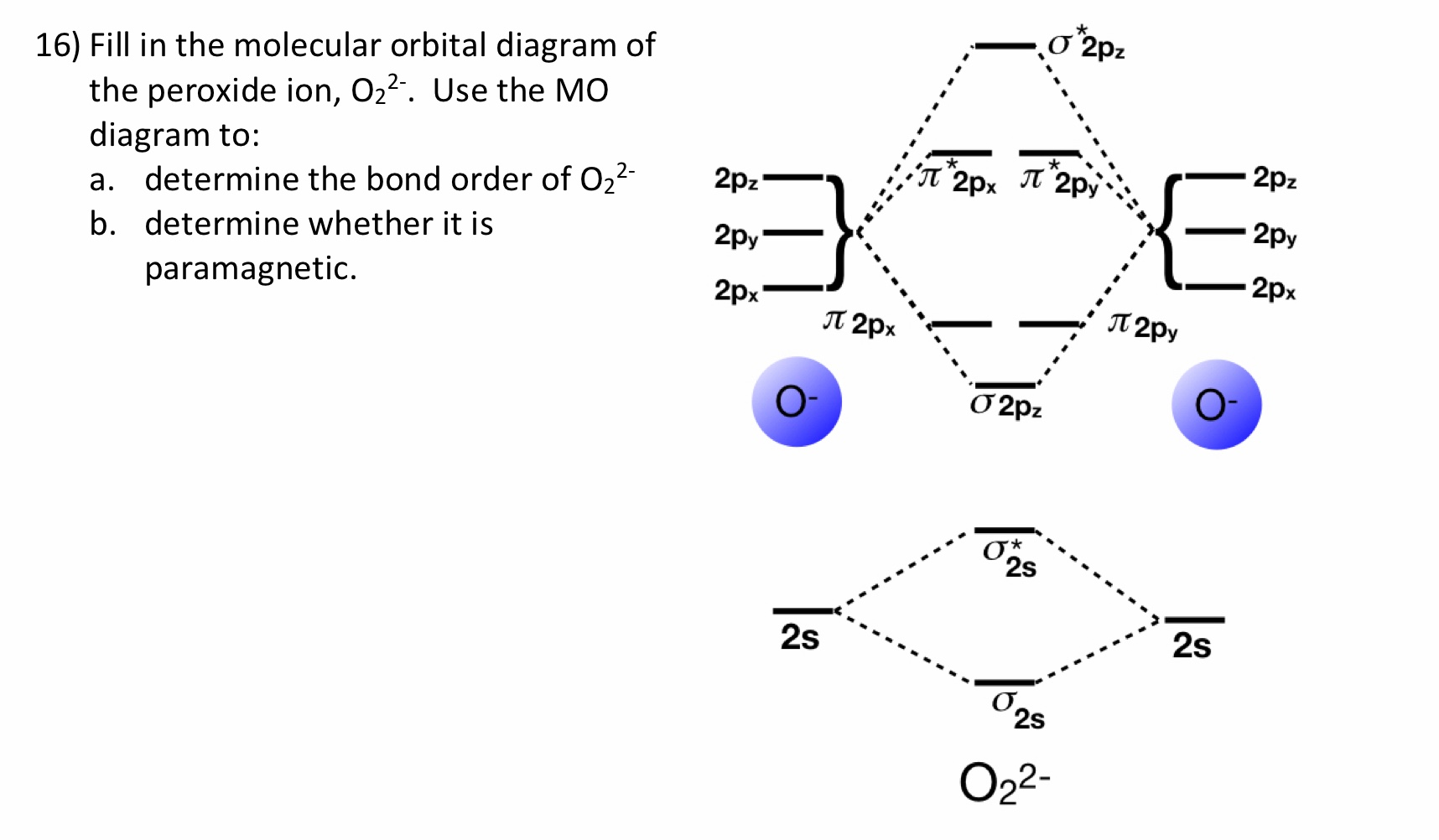
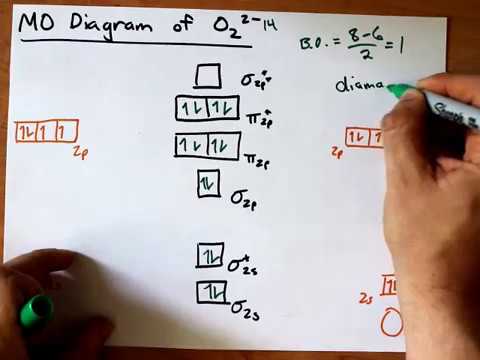
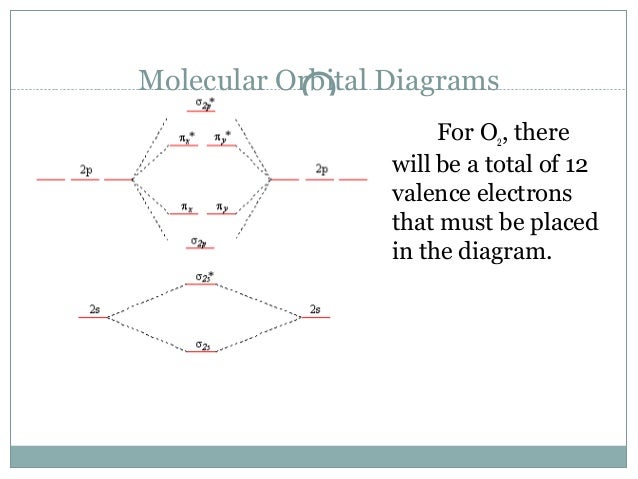
Jan 31, 2018 — Problem: Draw molecular orbital diagrams for O2-, O22-, and O2. ... good dot structures that correspond to each of these ions or molecules?1 answer · Top answer: First step is to find the total valence electrons for each molecule, O2 will appear to have 12 valence electrons (6+6 =12), O2- has 13 (12 +1) and O22- ...

Molecular orbital diagram for o2- ion
1918 (Venn's diagram is from 1904), named for English logician John Venn (1834-1923) of Cambridge, who explained them in the book "Symbolic Logic" (1881). 1834, introduced by English physicist and chemist Michael Faraday (suggested by the Rev. William Whewell, English polymath), coined from Greek ion, neuter present participle of ienai "go," from PIE root *ei- "to go." So called because ions move toward the electrode of opposite charge. 9.9.70: A Lewis structure obeying the octet rule can be drawn for O2 as fol... ... 9.9.84: FClO2 and F3ClO can both gain a fluoride ion to form ...
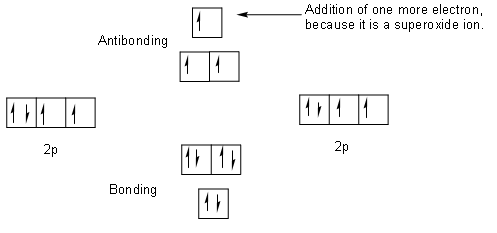
Molecular orbital diagram for o2- ion. Ions Are Created When Atoms Lose Or Gain Electrons ... What Is The Molecular Orbital Diagram For O2 ... Ion Formation In terms of determining if a substance is amphoteric, you should look at the oxygens and hydroxides on the molecule and if say an oxygen has only 1 bond and therefore an oxidation number of -1, it can accept a proton and and act as a base, but if it other spots it has hydrogens it can give, that could be one way to tell. Wouldn't that make one of the nitrogen have a +1 formal charge, leaving the oxygen with a -1 formal charge? If instead, the central nitrogen is double bonded to oxygen, wouldn't that minimize the formal charge of each atom and thus be the correct Lewis structure? Thanks in advance for the explanation! Mar 27, 2017 — The bond order is shown for the neutral oxygen. To figure it out for the positive ion simply remove one electron from the highest level, an antibonding orbital.5 answers · 31 votes: Hello! I actually just covered this question in my gen chem class this week. I have attached ...What is a bond order for 02-? - Quora9 answersMar 31, 2018What's the MOT diagram of O2 +2 ion? - Quora3 answersAug 25, 2017What is the molecular orbital diagram of O2 and F2 ...6 answersMar 12, 2017What is the molecular orbital diagram for oxygen ...4 answersAug 20, 2015More results from www.quora.com
Molecular Orbital Diagram For H2 And Bond Order h2 diagram molecular orbital bond order bonding hydrogen. ... Molecular Orbital Diagram For H2 diagram ... Mar 18, 2020 — 1: Molecular Orbital Energy-Level Diagrams for O2. With 12 valence electrons (6 from each O atom), there are only 2 electrons to place in the ( ... "relating to or consisting of molecules," by 1815, from molecule + -ar or else from French moléculaire or Modern Latin molecularis. Molecular biology is attested by 1950. Click here to get an answer to your question ✍️ In the molecular orbital diagram for O2^ + ion, the highest occupied orbital is:1 answer · Top answer: As it can be seen from the given structures that in the molecular orbital diagram for O2^ + ion, the highest occupied orbital is pi^* MO orbital.
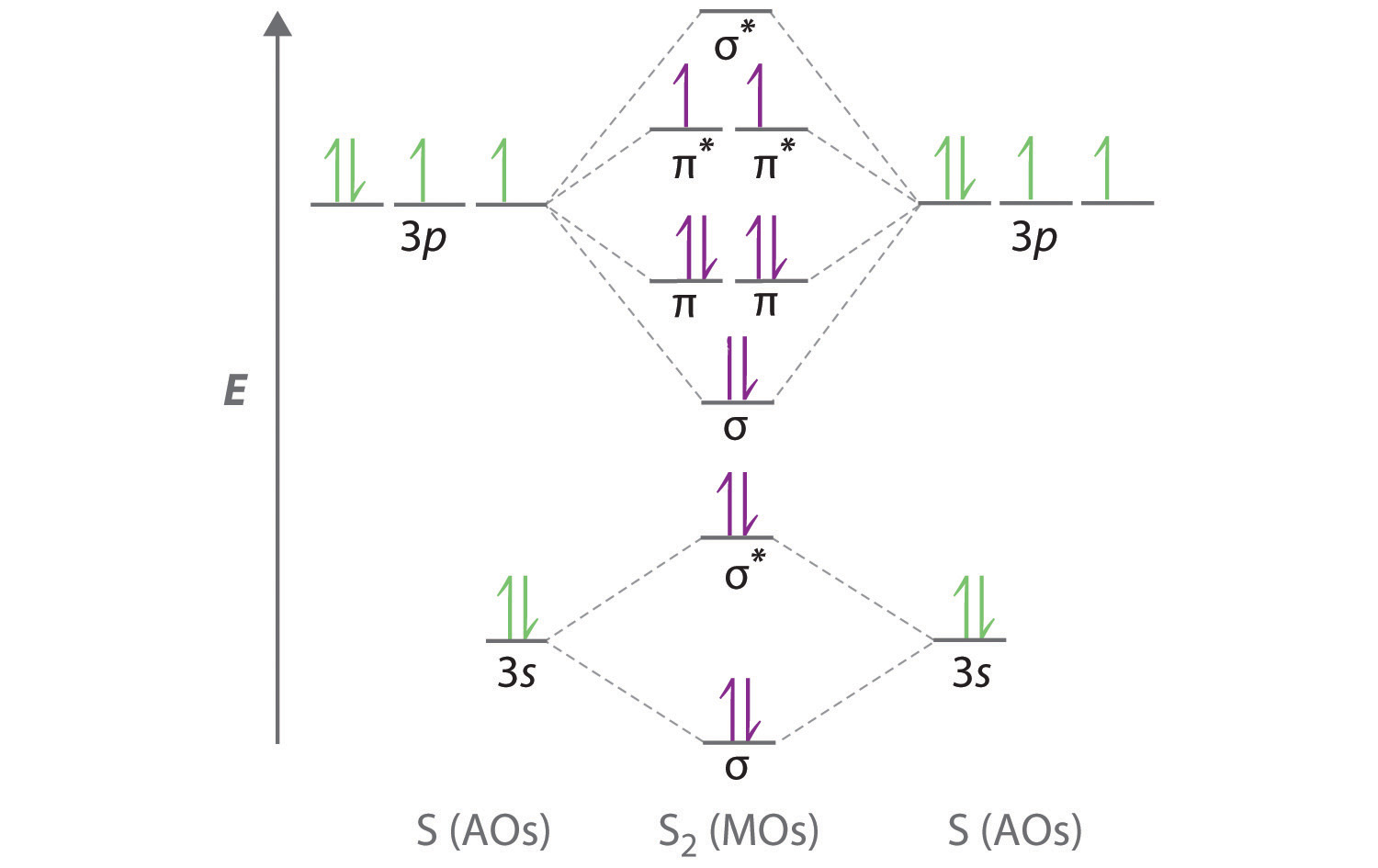
Linear Combination of Atomic Orbitals (LCAO), molecular orbitals of diatomic molecules, molecular orbital energy level diagrams of N2, O2 and F2 ... In each case, write the chemical equation for the reaction, determine its molecularity, and draw a proposed structure for the activated complex. word-forming element attached to verbs, making nouns of state, condition, or action, from French -ion or directly from Latin -ionem (nominative -io, genitive -ionis), common suffix forming abstract nouns from verbs. ... molecular orbital diagram for o2 molecule, write the orbital notation for iron, write orbital diagram for cd2, how to write shorthand orbital ...
also intraorbital, 1836, from intra- "within" + orbit (n.) + -al (1).
**Physical Chemistry** **Thermodynamics, Structure, and Change 10th Edition Solutions Peter Atkins, Julio de Paula** **ISBN-13: 9781429290197** Download the Solutions manual for this textbook **Order it via email: markrainsun"@"gmail(.)com** ​ **Table of Contents** **Foundations** A Matter B Energy C Waves **Part 1 Thermodynamics** **1. The properties of gases** Topic 1A The perfect gas Topic 1B The kinetic model Topic 1C Real gases **Impact** …O...
What are molecular orbital theory and valence bond theory? What does molecular orbital theory explain that valence bond theory does not?
Old English for "before, in the sight of, in the presence of; as far as; during, before; on account of, for the sake of; in place of, instead of," from Proto-Germanic *fur "before; in" (source also of Old Saxon furi "before," Old Frisian for, Middle Dutch vore, Dutch voor "for, before;" German für "for;" Danish for "for," før "before;" Gothic faur "for," faura "before"), from PIE root *per- (1) "forward," hence "in front of, before," etc. From late Old English as "in favor of." For and fore differentiated gradually in Middle English. For alone as a conjunction, "because, since, for the reason that; in order that" is from late Old English, probably a shortening of common Old English phrases such as for þon þy "therefore," literally "for the (reason) that."
Construct The Molecular Orbital Diagram For He2 And Then orbital he2 molecular diagram h2 bond order mo construct stable li identify then.
1540s, "of or pertaining to the eye socket;" 1839 with reference to heavenly bodies; from orbit (n.) + -al (1).
May 19, 2014 — Answer. O2+ is more stable than O2-.Because According to molecular orbital theory O2+ has 15 electrons &it has one electron in antibonding ...
Drawing the MO diagram for O2 and F2 ... Topic: Combination Reactions, Decomposition, Nomenclature of polyatomic ions, Definition and nomenclature of ...
You can also explain the octet rule and its limitations, draw Lewis structures of simple molecules and the formation of different types of bonds.
1550s, "be taken or regarded as," also "be in favor of," from go (v.) + for (adv.). Meaning "attack, assail" is from 1880. Go for broke is from 1951, American English colloquial.

Evaluating Chemical Bonding In Dioxides For The Development Of Metal Oxygen Batteries Vibrational Spectroscopic Trends Of Dioxygenyls Dioxygen Supe Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 C8cp04652b
prefix usually meaning "away, opposite, completely," from Old English for-, indicating loss or destruction, but in other cases completion, and used as well with intensive or pejorative force, from Proto-Germanic *fur "before, in" (source also of Old Norse for-, Swedish för-, Dutch ver-, Old High German fir-, German ver-); from PIE *pr-, from root *per- (1) "forward," hence "in front of, before, toward, near, against." In verbs the prefix denotes (a) intensive or completive action or process, or (b) action that miscarries, turns out for the worse, results in failure, or produces adverse or opposite results. In many verbs the prefix exhibits both meanings, and the verbs frequently have secondary and figurative meanings or are synonymous with the simplex. [Middle English Compendium] Probably originally in Germanic with a sense of "forward, forth," but it spun out complex sense developments in the historical languages. Disused as a word-forming element in Modern English. Ultimately from the same root as fore (adv
Organometallics and Related Molecules for Energy Conversion
9.9.70: A Lewis structure obeying the octet rule can be drawn for O2 as fol... ... 9.9.84: FClO2 and F3ClO can both gain a fluoride ion to form ...
1834, introduced by English physicist and chemist Michael Faraday (suggested by the Rev. William Whewell, English polymath), coined from Greek ion, neuter present participle of ienai "go," from PIE root *ei- "to go." So called because ions move toward the electrode of opposite charge.
1918 (Venn's diagram is from 1904), named for English logician John Venn (1834-1923) of Cambridge, who explained them in the book "Symbolic Logic" (1881).

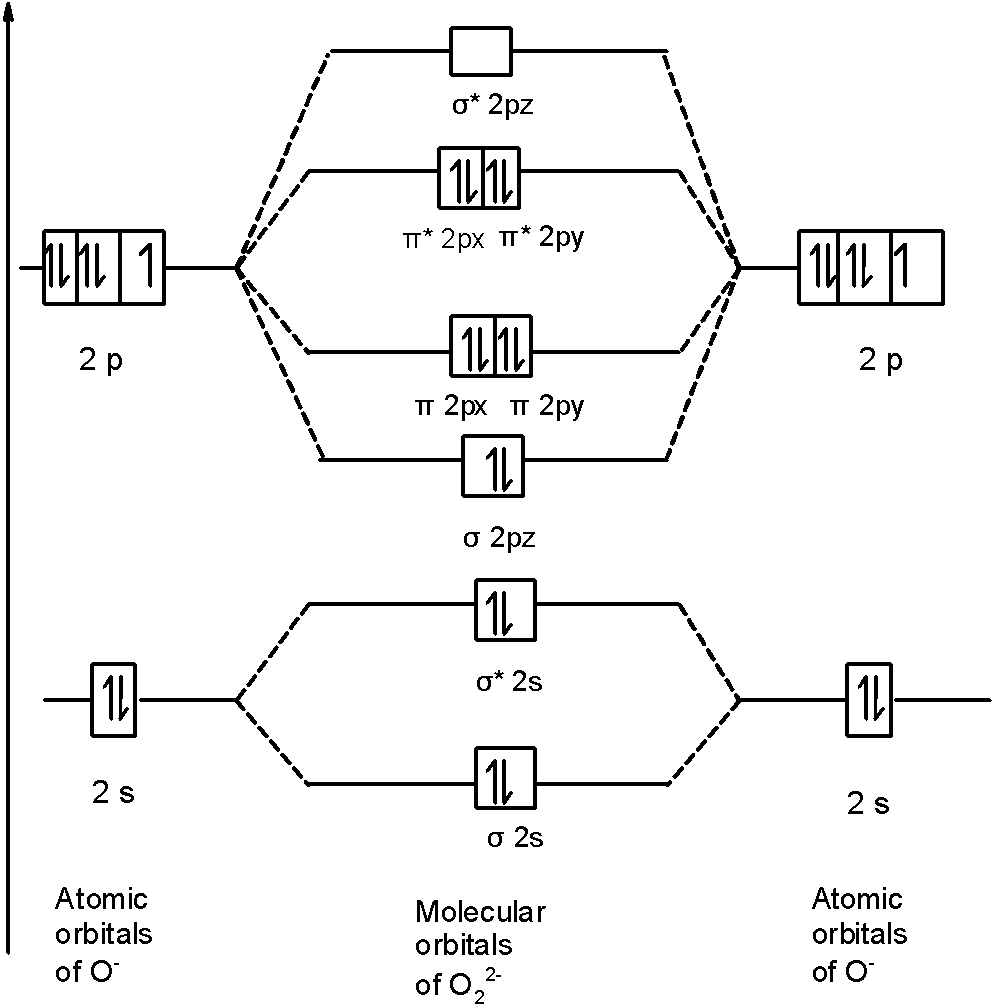
Calculate The Number Of Unpaired Electrons In O2 And O2 2 Ions By Drawing Molecular Orbital Brainly In

Based On The Mo Diagrams For O 2 O 2 And O 2 Answer The Following 1 Is O 2 Paramagnetic Or Diamagnetic 2 Which Will Have The Shortest Bond Length 3 Which Will Have The

Draw The Molecular Orbital Energy Diagram For Oxygen Molecule O2 And Show That I It Has A Double Bond Ii It Has Paramagnetic Character From Chemistry Chemical Bonding And Molecular Structure Class 11 Cbse

Draw The Molecular Orbital Diagram Of O2 And Calculate The Bond Order Is O2 Diamagnetic Or Paramagnetic Explain Your Answer Study Com
Molecular Orbital Theory Chemistry Encyclopedia Structure Number Molecule Atom Bond Order Multiple Bonds

















0 Response to "40 molecular orbital diagram for o2- ion"
Post a Comment