37 look at the following reaction energy diagram.
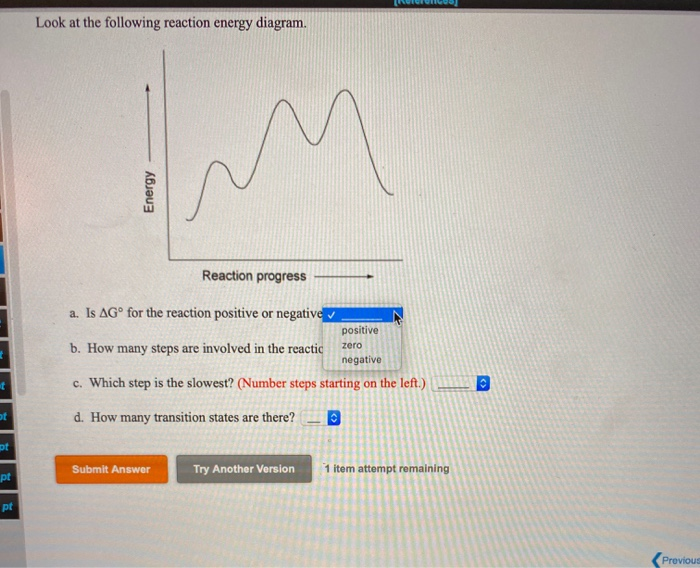
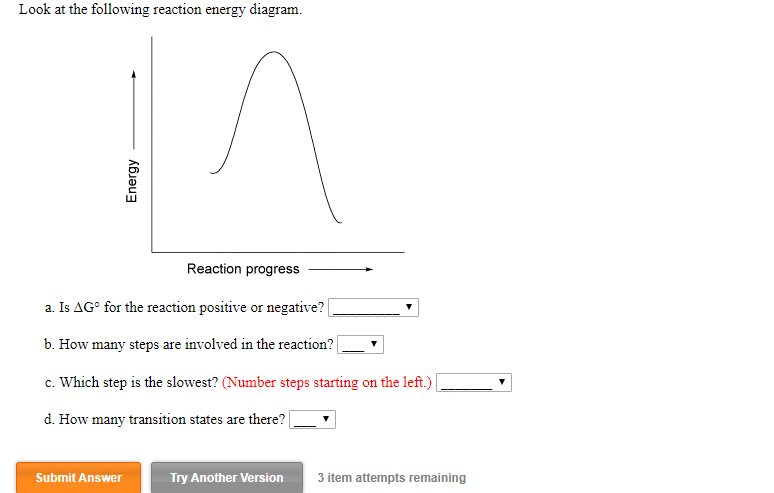
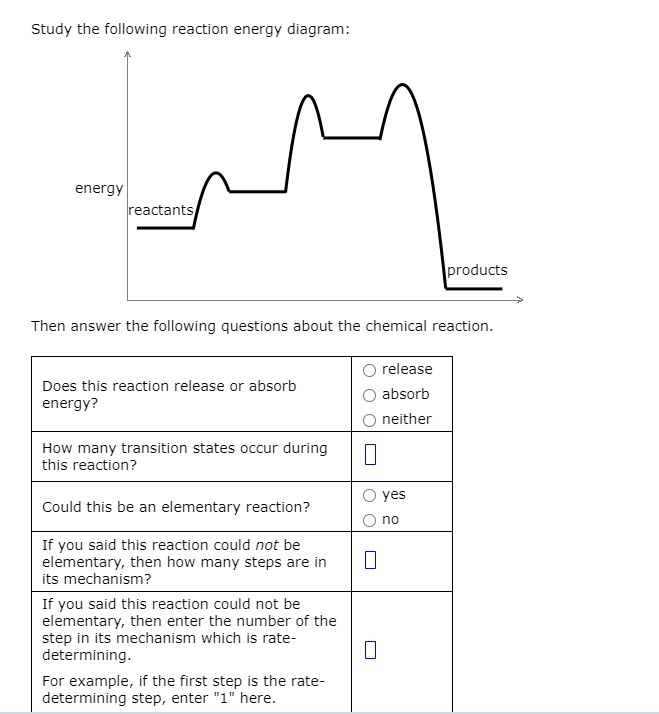
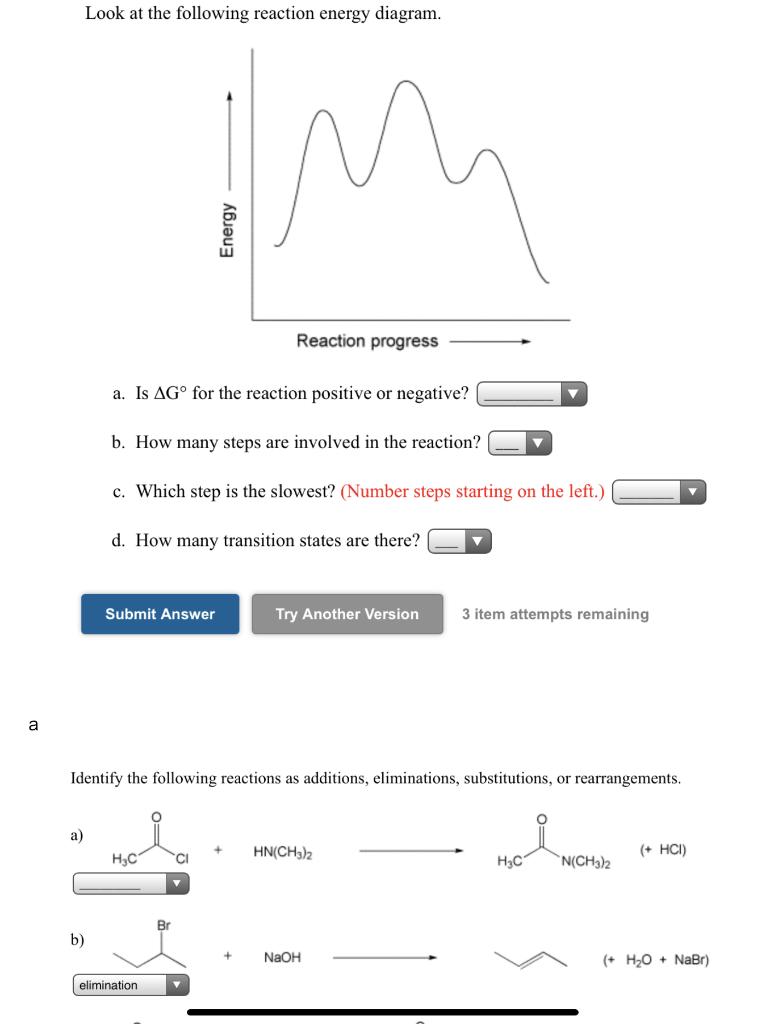
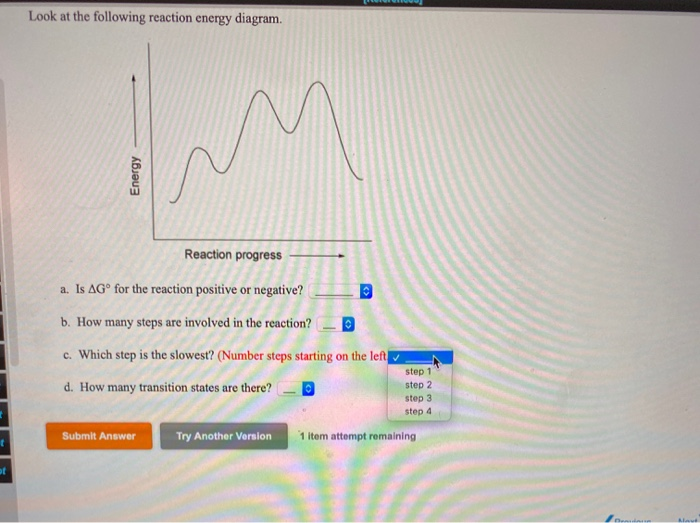
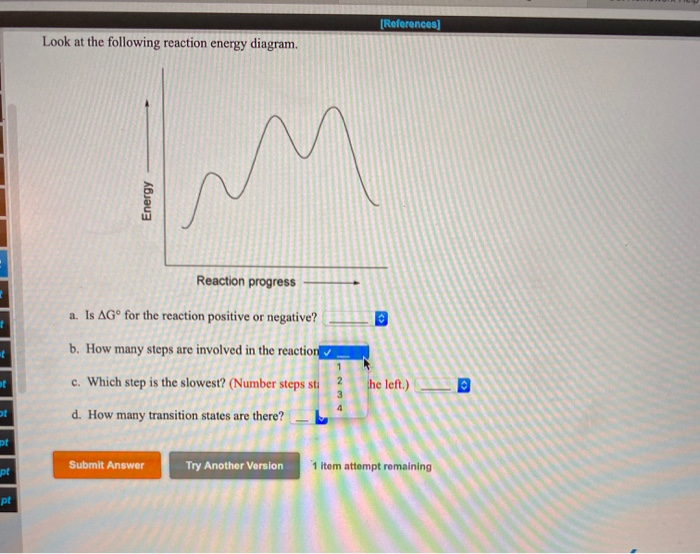
Question: Look at the following reaction energy diagram Energy Reaction progress a Is AG" for the reaction positive or negative? b. How many steps are involved in the reaction? c. Which step is the slowest? (Number steps starting on the left.) 4. How many transition states are there! B Submit Answer Try Another Version hematempts remaining ... Look at the following reaction energy diagram Reaction progress a. Is ΔGo for the reaction positive or negative? b. How many steps are involved in the reaction?Y c. Which step is the slowest? (Number steps starting on the left.) d. How many transition states are there? Question: Look at the following reaction energy diagram Reaction progress a ...
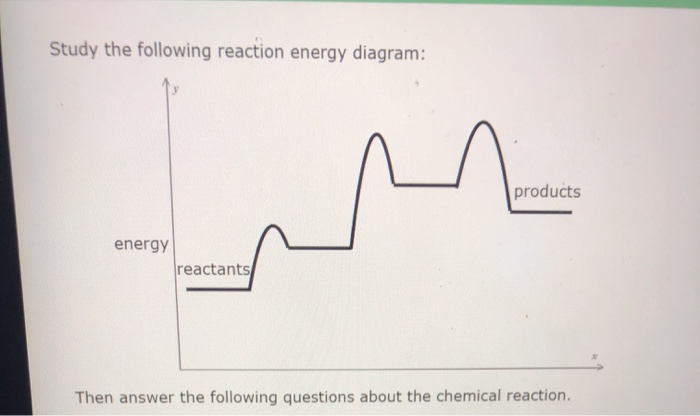
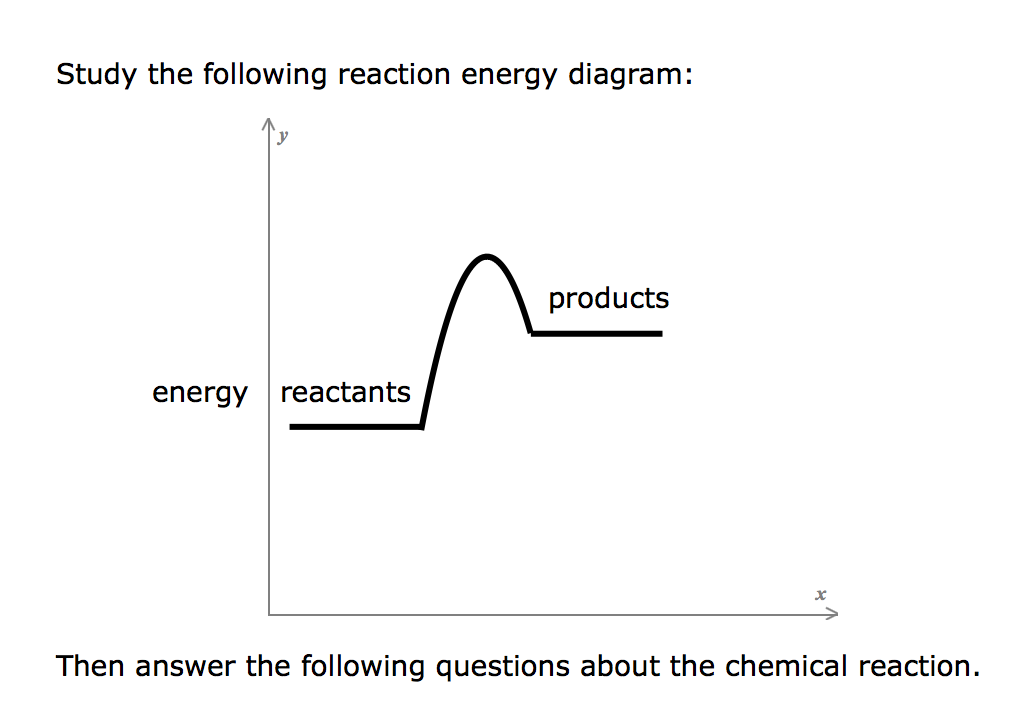
Transcribed image text: Study the following reaction energy diagram: energy reactants products Then answer the following questions about the chemical reaction. Does this reaction release or absorb energy? release absorb neither 0 How many transition states occur during this reaction? yes Could this be an elementary reaction? no If you said this reaction could not be elementary, then how many ...
Look at the following reaction energy diagram.
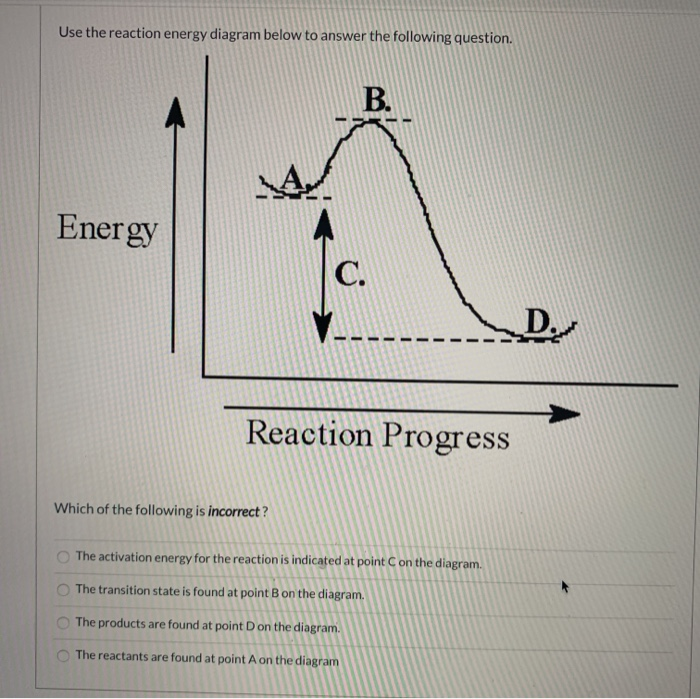
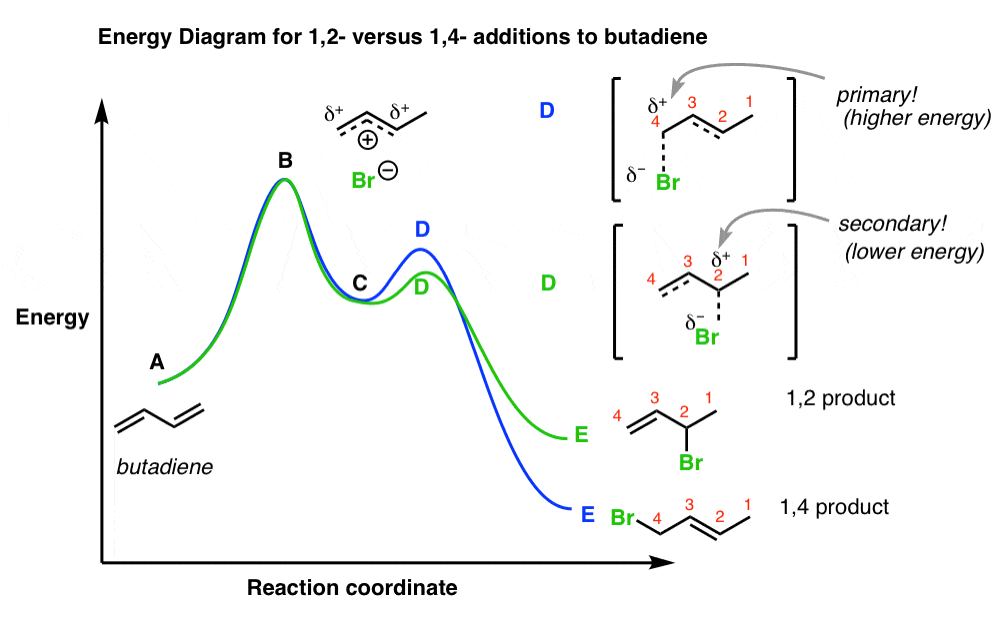
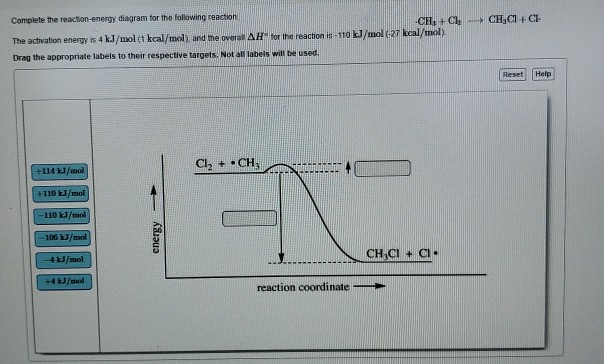
Look at the following reaction energy diagram. Is for the reaction positive or negative? How many steps are involved in the reaction? Which steps is the slowest? How many transition states are there? Question: Look at the following reaction energy diagram. Is for the reaction positive or negative? How many steps are involved in the reaction? Look at the following diagram, which depicts the energy diagram for a chemical reaction. Which point on the diagram represents the point at which the transition state exists? a) A b) B c) C d) D Question 5 Which of the following statements regarding the use of catalysts is false? a) Catalysts ... Alternate ISBN: 9781305638716, 9781305686465. Organic Chemistry (9th Edition) Edit edition Solutions for Chapter 6 Problem 17E: Look at the following energy diagram: (a) Is ΔG° for the reaction positive or negative? Label it on the diagram. (b) How many steps are involved in the reaction?
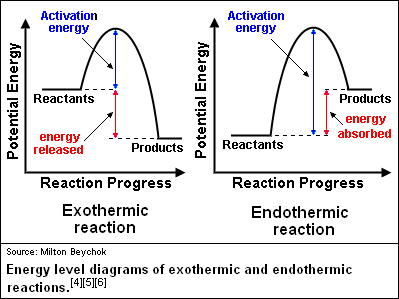
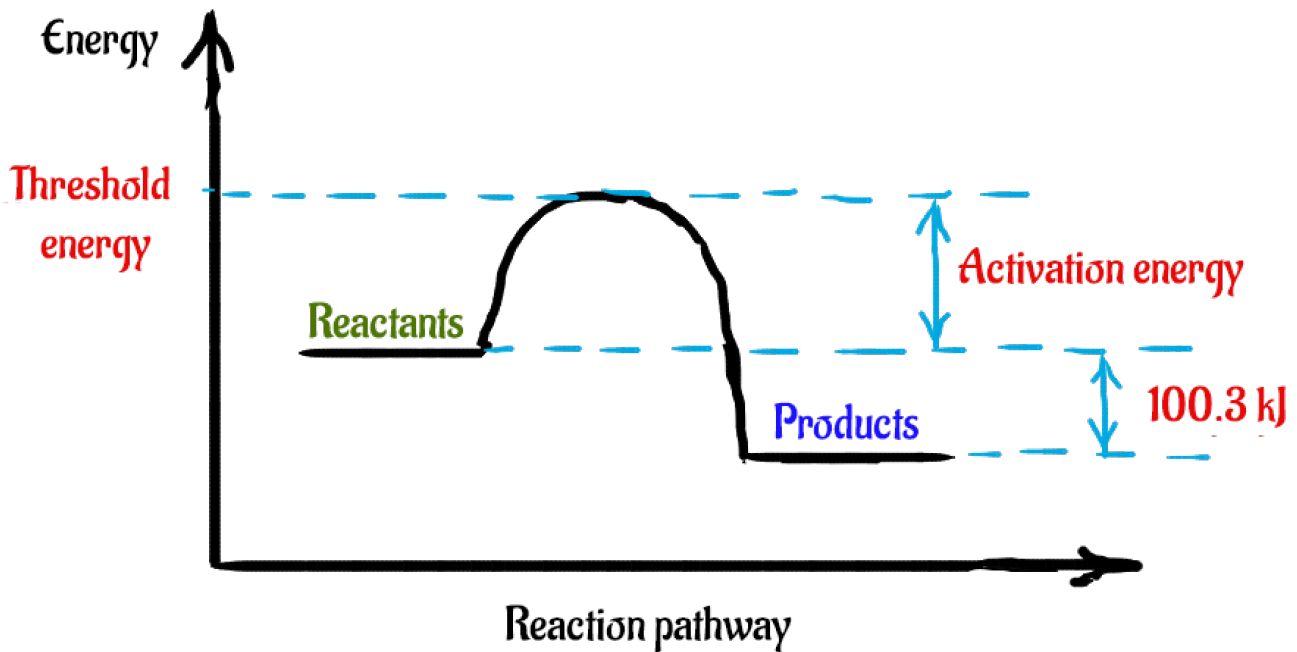
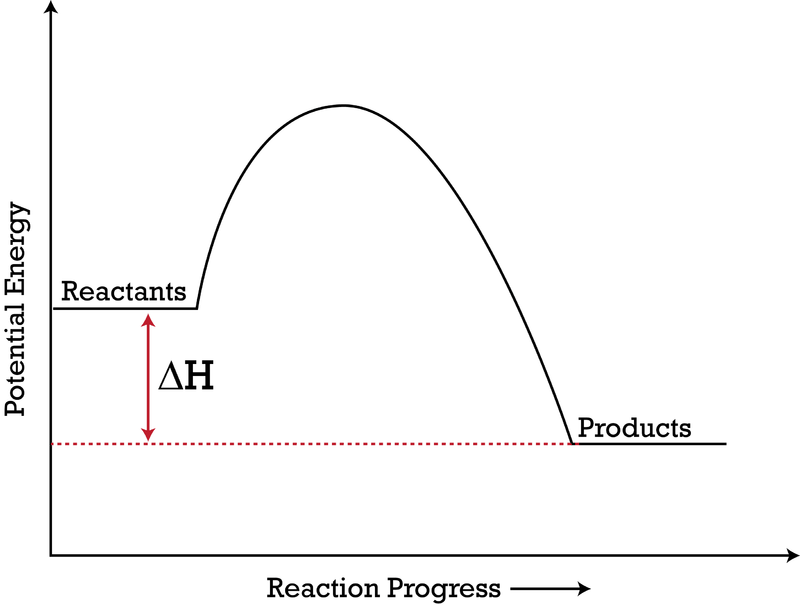
Look at the following reaction energy diagram.. Look at the following energy diagram. Select the appropriate description Cart CH =-0.45 kcal molec Products +AH Enthalpy Congo Reactants ... In an exothermic reaction, the energy of products are lesser than the reactants energy. Thus, the change in enthalpy is negative. Hence, the correct option is, the reaction is endothermic and the products ... Consider the following diagrams which show the progress for the reaction A (blue) ⇌ B (red). The equilibrium constant (K) for this reaction is 0.8. At which point does the reaction reach equilibrium? The equilibrium constant is the products divided by the reactants. A K value of 0.8 is consistent with image choice C where the ratio of product ... We review their content and use your feedback to keep the quality high. a) G for this reaction is positive …. View the full answer. Transcribed image text: Look at the following reaction energy diagram. Energy Reaction progress a. Is AGº for the reaction positive or negative positive b. How many steps are involved in the reactic zero negative c. Science. Chemistry. Chemistry questions and answers. Look at the following reaction energy diagram Is Delta G degree for the reaction positive or negative? How many steps are involved m the reaction? Which step is the slowest? (Number steps starting on the left). How many transition states are there?
Alternate ISBN: 9781305638716, 9781305686465. Organic Chemistry (9th Edition) Edit edition Solutions for Chapter 6 Problem 17E: Look at the following energy diagram: (a) Is ΔG° for the reaction positive or negative? Label it on the diagram. (b) How many steps are involved in the reaction? Look at the following diagram, which depicts the energy diagram for a chemical reaction. Which point on the diagram represents the point at which the transition state exists? a) A b) B c) C d) D Question 5 Which of the following statements regarding the use of catalysts is false? a) Catalysts ... Look at the following reaction energy diagram. Is for the reaction positive or negative? How many steps are involved in the reaction? Which steps is the slowest? How many transition states are there? Question: Look at the following reaction energy diagram. Is for the reaction positive or negative? How many steps are involved in the reaction?
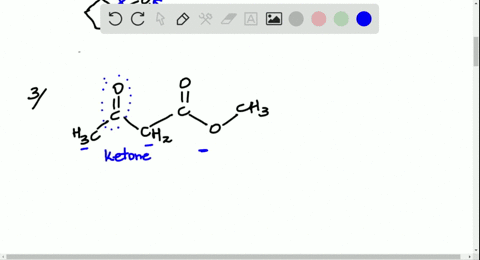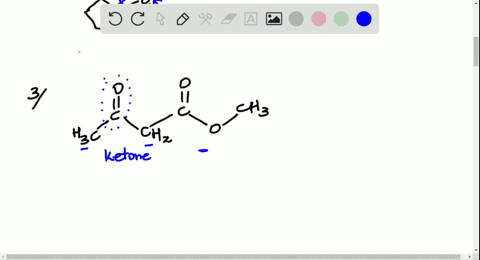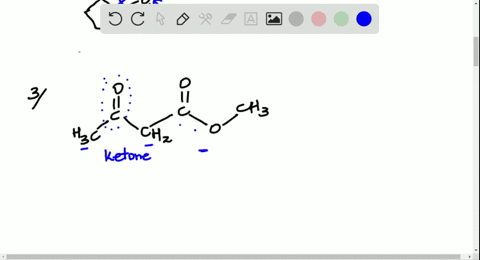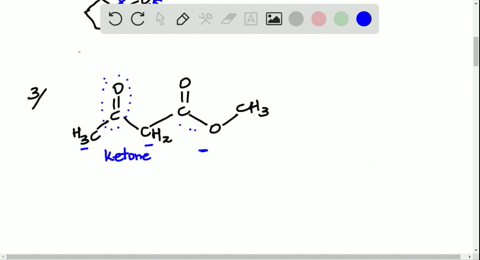
Solved Look At The Following Energy Diagram For An Enzyme Catalyzed Reaction Figure Can T Copy A How Many Steps Are Involved B Which Step Is Most Exergonic C Which Step Is Slowest

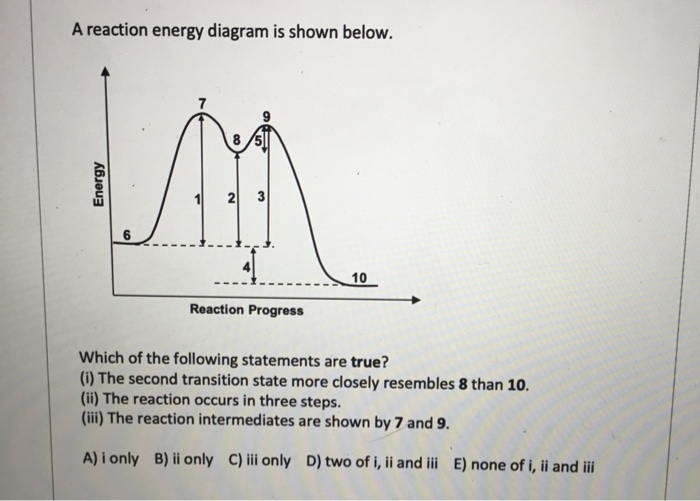
Which One Of The Following Reaction Energy Diagrams Best Represents A Reaction In The Reverse Direction I E The Most Endothermic Reaction

7 For The Energy Diagram Shown Below Which Letter 10 Looking Ahead Predict The Product Of Homeworklib

How Would This Reaction Look Like In A Potential Energy Diagram Sn1 Reaction Chemistry Stack Exchange

Please Answer In Details Showing All The Steps Thank You Look At The Following Reaction Toluene Homeworklib

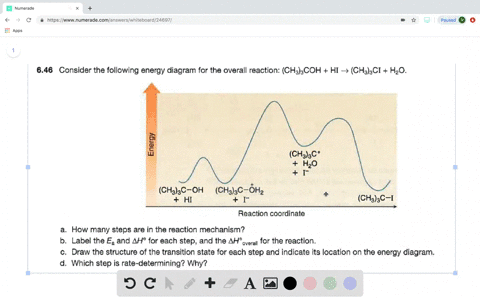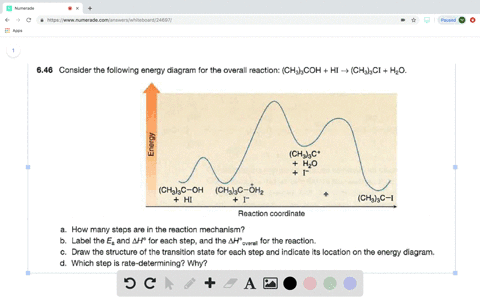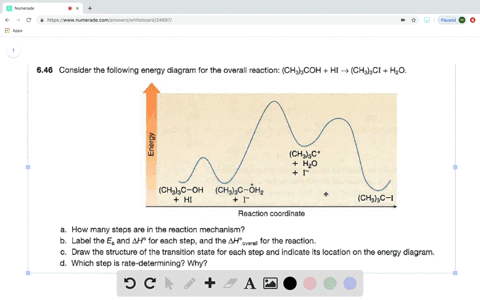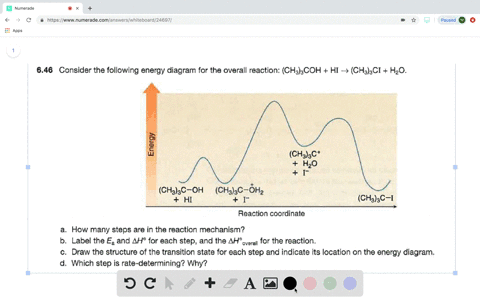
2018 Chapter6 Lectureproblems Key Pdf Chapter 6 Lecture Problems Key 1 Consider The Following Energy Diagram A Is U0394g U00b0 For The Reaction Positive Or Course Hero

The Following Diagram Shows The Energy Of A Reaction As The Reaction Progresses Label Each Of Homeworklib

10 Looking Ahead Predict The Product Of The Following Reaction Sequence 7 For The Energy Diagram Homeworklib







0 Response to "37 look at the following reaction energy diagram."
Post a Comment