37 lewis dot diagram for iodine
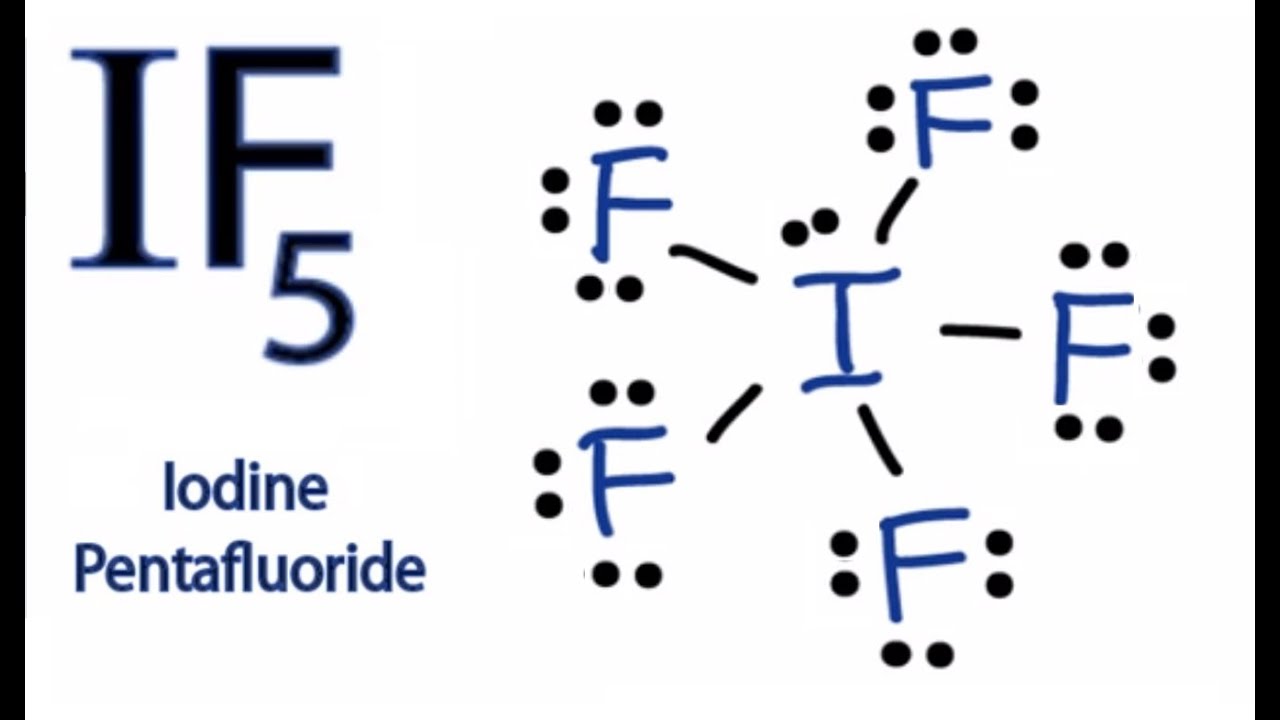
Atomic Structure of Iodine ... Electron Configuration: 1s2 2s2p6 3s2p6d10 4s2p6 ... Iodine is below Period Two on the periodic table so it can have an expanded octet (hold more than eight valence electrons). In the Lewis structure for IF5 you' ...25 Oct 2016 · Uploaded by Wayne Breslyn
28 Apr 2021 — Iodine has seven valence electrons, because it is in group seven on the periodic table and is a halogen. The dot structure would be the element ...
Lewis dot diagram for iodine
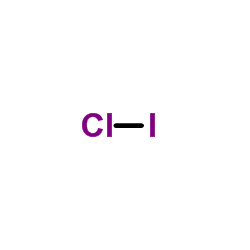
1:08Because the Iodide ion (I-) has an extra electron (the negative sign denotes an extra electron) we need to add ...22 Nov 2018 · Uploaded by Wayne Breslyn A video explanation of how to draw the Lewis Dot Structure for Iodine Monofluoride, along with information about the compound including Formal Charges, Polar... Topic: Lewis Dot Diagrams for Ionic Compounds. Do Now: Identify ionic compounds. CO. 2 MgCl. 2 NH. 4. ClNaOH. NH. 3 CH. 4 CuSO. 4 HF. Magnesium’s outer shell is now empty. Fluorine’s outer shell is now full. ... How many iodine ions do you need to make a neutral compound? 2 ...
Lewis dot diagram for iodine. 1:13Once we know how many valence electrons there are in IF we can distribute them around the central atom with ...20 Aug 2019 · Uploaded by MagnetsAndMotors A video explanation of how to draw the Lewis Dot Structure for Iodine Pentabromide, along with information about the compound including Formal Charges, Polar... 1 answerHint: The electron dot diagram is the diagram which clearly shows the bonding between the atoms of a molecule along with the lone pairs of electrons, ... The lewis dot diagram for platinum is a diagram showing bonds electrons of the platinum atom within a molecule. That means it has 7 valence electrons so we have 7. Iodine is in group 7 of the periodic table. Iodine is in group 17 on the periodic table meaning that iodine needs only a single electron to become chemically stable.
Comprehensive information for the element Iodine - I is provided by this page including scores of properties, Atomic Structure of Iodine Electron Dot Model . The left diagram shows a Lewis dot structure of sodium with. Likewise, they can be used .. The left shows an iodine atom with one lone pair. When we write the . The lewis dot diagram for platinum is a diagram showing bonds electrons of the platinum atom within a molecule. Were going to draw the lewis structure for i2 iodine gas a very pretty purple gas. The shape of a molecule. That means it has 7 valence electrons so we have 7. The iodine atom from group vii has 7 valence electrons. Draw a Lewis dot diagram for an iodine molecule. 2. Draw a Lewis dot diagram for a hydrogen molecule. 3. True or false: When drawing a covalent bond, it is proper to use a line connect atoms and a line represents 2 electrons. 4. In your own words, describe the difference between a bond that is nonpolar covalent and polar covalent. Cite one ... A step-by-step explanation of how to draw the I Lewis Dot Structure.For the I structure use the periodic table to find the total number of valence electrons ...
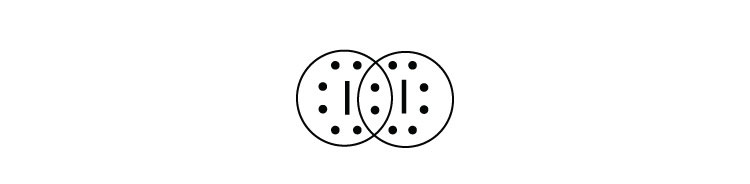
1:36A step-by-step explanation of how to draw the I2 Lewis Dot Structure (Iodine Gas).For the I2 structure use the ...4 May 2013 · Uploaded by Wayne Breslyn Lewis dot diagram for iodine. Wiki User. ∙ 2009-06-12 05:08:19. See Answer. Best Answer. Copy. Iodine is a diatomic molecule so it's molecules are paired as I2 Iodine has 7 electrons in it's ... A step-by-step explanation of how to draw the IF Lewis Dot Structure.For the IF structure use the periodic table to find the total number of valence electron... Topic: Lewis Dot Diagrams for Ionic Compounds. Do Now: Identify ionic compounds. CO. 2 MgCl. 2 NH. 4. ClNaOH. NH. 3 CH. 4 CuSO. 4 HF. Magnesium’s outer shell is now empty. Fluorine’s outer shell is now full. ... How many iodine ions do you need to make a neutral compound? 2 ...
A video explanation of how to draw the Lewis Dot Structure for Iodine Monofluoride, along with information about the compound including Formal Charges, Polar...
1:08Because the Iodide ion (I-) has an extra electron (the negative sign denotes an extra electron) we need to add ...22 Nov 2018 · Uploaded by Wayne Breslyn
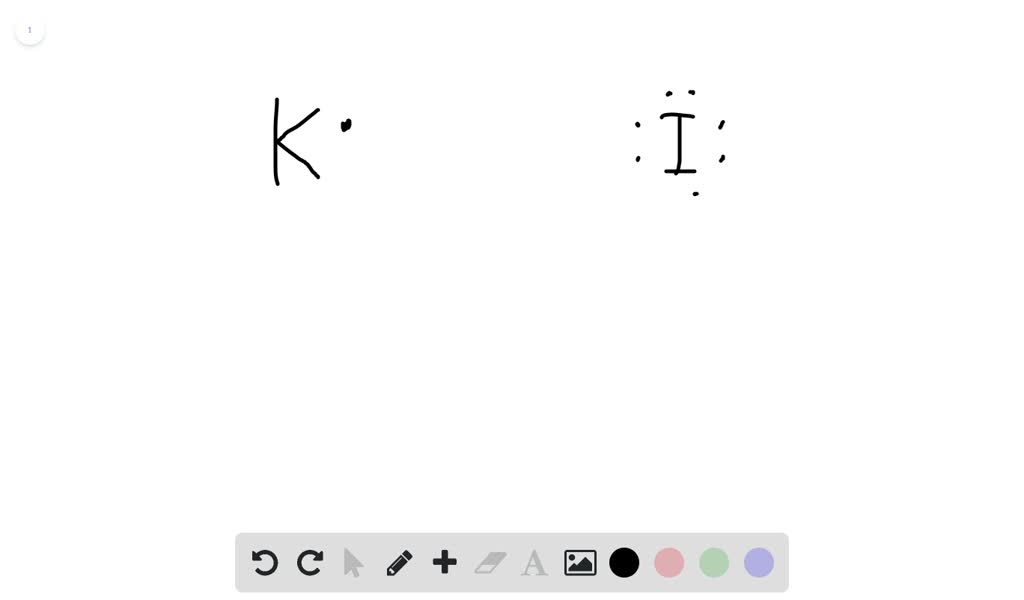
Solved Using Electron Dot Structures Diagram The Formation Of An Ionic Bond Between Potassium And Iodine

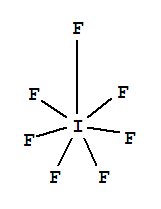
Yodium Pentafluoride Struktur Lewis Iodine Heptafluoride Arsenic Pentafluoride Lainnya Sudut Teks Lain Lain Png Pngwing

Chemistry Lewis Structure Iodine Heptafluoride Triiodide Lewis Acids And Bases Polyiodide Electron Vsepr Theory Molecular Geometry Polyhalogen Ions Lewis Structure Iodine Heptafluoride Triiodide Png Pngwing




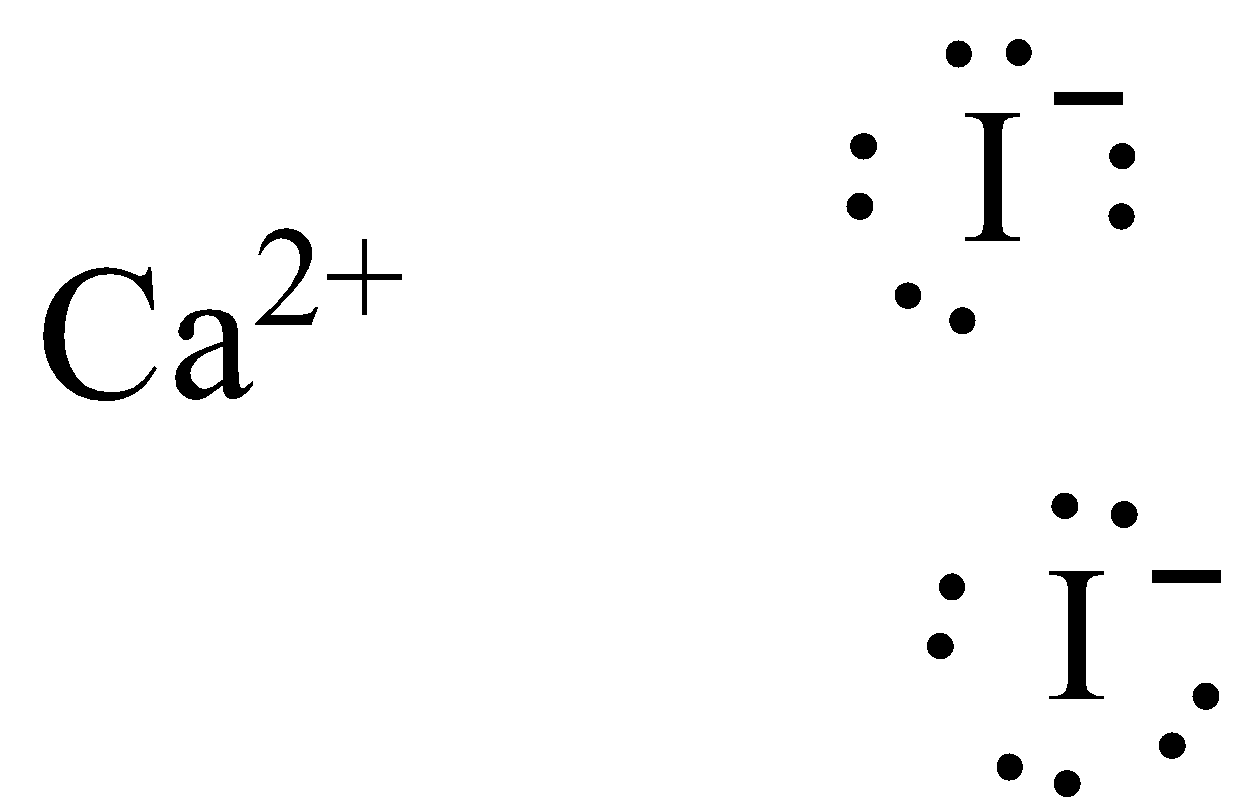


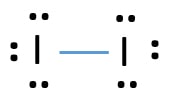


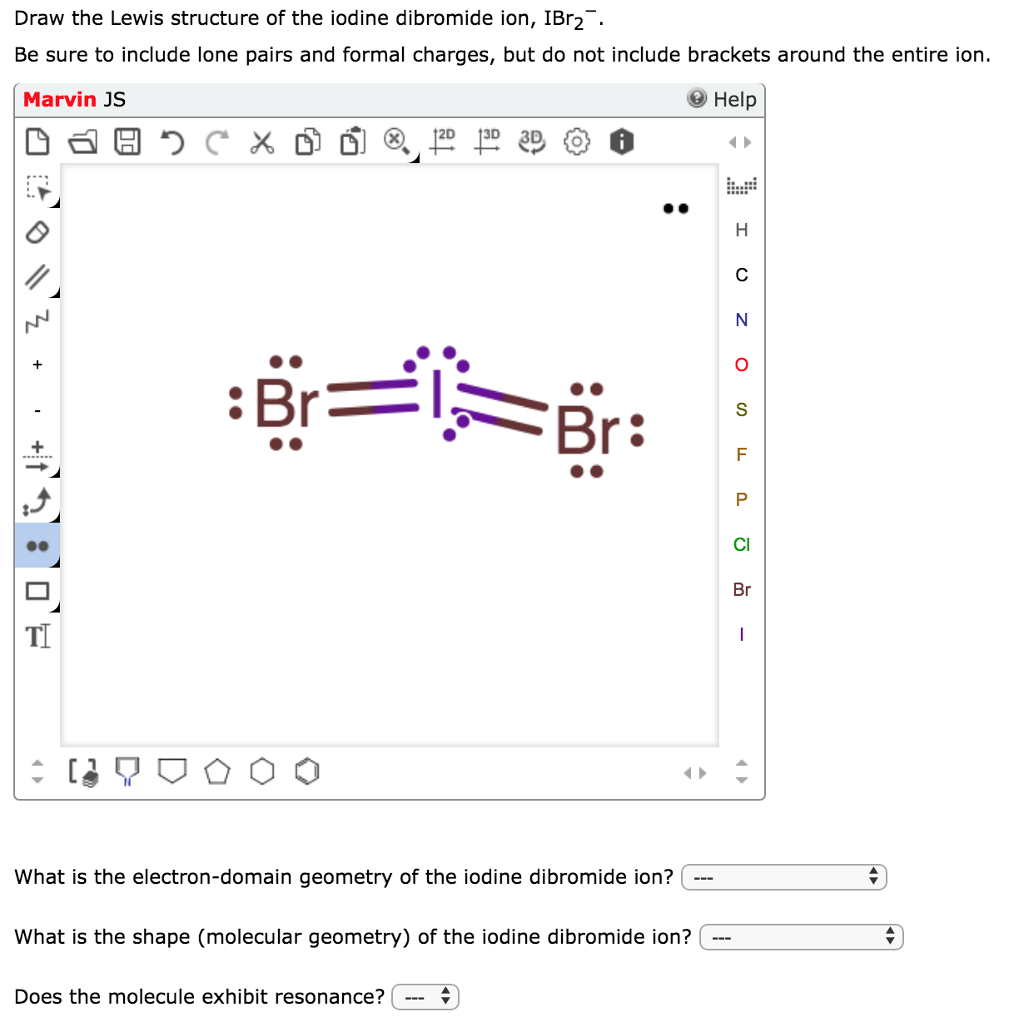









0 Response to "37 lewis dot diagram for iodine"
Post a Comment